Oral anticoagulation in device-detected atrial fibrillation: effects of age, sex, cardiovascular comorbidities, and kidney function on outcomes in the NOAH-AFNET 6 trial
- PMID: 38591192
- PMCID: PMC11107119
- DOI: 10.1093/eurheartj/ehae225
Oral anticoagulation in device-detected atrial fibrillation: effects of age, sex, cardiovascular comorbidities, and kidney function on outcomes in the NOAH-AFNET 6 trial
Keywords: Anticoagulation; Atrial fibrillation; Atrial high-rate episodes; Bleeding; CHA2DS2-VASc score; Device-detected atrial fibrillation; Kidney function; NOAH-AFNET 6; Stroke.
Figures
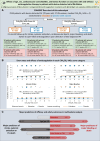
References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 - DOI - PubMed
-
- Becher N, Toennis T, Bertaglia E, Blomstrom-Lundqvist C, Brandes A, Cabanelas N, et al. . Anticoagulation with edoxaban in patients with long atrial high-rate episodes ≥ 24 h. Eur Heart J 2024;45:837–49. 10.1093/eurheartj/ehad771 - DOI
-
- McIntyre WF, Benz AP, Becher N, Healey JS, Granger CB, Rivard L, et al. . Direct oral anticoagulants for stroke prevention in patients with device-detected atrial fibrillation: a study-level meta-analysis of the NOAH-AFNET 6 and ARTESiA trials. Circulation 2024;149:981–8. 10.1161/CIRCULATIONAHA.123.067512 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

