Midfacial toddler excoriation syndrome (MiTES): case series, diagnostic criteria and evidence for a pathogenic mechanism
- PMID: 38591490
- PMCID: PMC11324070
- DOI: 10.1093/bjd/ljae151
Midfacial toddler excoriation syndrome (MiTES): case series, diagnostic criteria and evidence for a pathogenic mechanism
Abstract
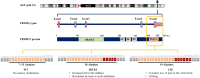
Background: PRDM12 polyalanine tract expansions cause two different disorders: midfacial toddler excoriation syndrome (MiTES; itch with normal pain sensation associated with 18 homozygous alanines (18A); and congenital insensitivity to pain (CIP) with normal itch associated with 19 homozygous alanines (19A). Knowledge of the phenotype, genotype and disease mechanism of MiTES is incomplete. Why 18A vs. 19A PRDM12 can cause almost opposite phenotypes is unknown; no other polyalanine or polyglutamine tract expansion disease causes two such disparate phenotypes.
Objectives: To assess the genotype and phenotype of nine new, nine atypical and six previously reported patients diagnosed with MiTES.
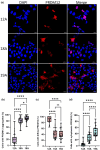
Methods: Using cell lines with homozygous PR domain zinc finger protein 12 (PRDM12) containing 12 alanines (12A; normal), 18A (MiTES) and 19A (CIP), we examined PRDM12 aggregation and subcellular localization by image-separation confocal microscopy and subcellular fractionation Western blotting.
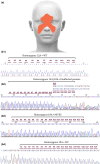
Results: MiTES presents in the first year of life; in all cases the condition regresses over the first decade, leaving scarring. The MiTES phenotype is highly distinctive. Features overlapping with PRDM12 CIP are rarely found. The genotype-phenotype study of the PRDM12 polyalanine tract shows that having 7-15 alanines is normal; 16-18 alanines is associated with MiTES; 19 alanines leads to CIP; and no clinically atypical cases of MiTES had a polyalanine tract expansion. PRDM12 aggregation and subcellular localization differed significantly between 18A and normal 12A cell lines and between 18A and 19A cell lines. MiTES is a new protein-aggregation disease.
Conclusions: We provide diagnostic criteria for MiTES and improved longitudinal data. MiTES and CIP are distinct phenotypes, despite their genotypes varying by a single alanine in the PRDM12 polyalanine tract. We found clear distinctions between the cellular phenotypes of normal, MiTES and CIP cells. We hypothesize that the developmental environment of the trigeminal ganglion is unique and critically sensitive to pre- and postnatal levels of PRDM12.
Plain language summary
Midfacial toddler excoriation syndrome (MiTES) causes facial itching and scratching in babies during their first year of life. MiTES tends to improve over the time period of approximately 10 years, but it can leave scars. Congenital insensitivity to pain (CIP) is a condition where a person cannot feel pain and is present from birth. This study looked at two conditions: MiTES and CIP. We specifically investigated changes in a gene called PRDM12, focusing on a part of the gene called the polyalanine tract – a sequence of many alanines (alanine is a type of amino acid). We discovered that the normal range for this sequence is between 7 and 15 alanines. If there are 16 to 18 alanines, it is associated with MiTES and causes the PRDM12 protein to clump together inside the cell. However, if there are 19 alanines, it leads to CIP, and the PRDM12 protein clumps together and moves to the cytoplasm, where it should not be. We found new evidence to suggest that MiTES is a disease where proteins clump together. Overall, our study findings show that despite there only being a small change in the same gene, MiTES and CIP are very different conditions.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Association of Dermatologists.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures

Comment in
-
MiTES: itch or pain?Br J Dermatol. 2024 Aug 14;191(3):323-324. doi: 10.1093/bjd/ljae198. Br J Dermatol. 2024. PMID: 38736256 No abstract available.
Similar articles
-
Midface toddler excoriation syndrome (MiTES) can be caused by autosomal recessive biallelic mutations in a gene for congenital insensitivity to pain, PRDM12.Br J Dermatol. 2018 Nov;179(5):1135-1140. doi: 10.1111/bjd.16893. Epub 2018 Sep 16. Br J Dermatol. 2018. PMID: 29949203
-
A case of mid-face toddler excoriation syndrome (MiTES).Pediatr Dermatol. 2020 Mar;37(2):345-346. doi: 10.1111/pde.14081. Epub 2020 Jan 12. Pediatr Dermatol. 2020. PMID: 31930564
-
Structural and functional annotation of PR/SET Domain (PRDM) protein family: In-silico study elaborating role of PRDM12 mutation in congenital insensitivity to pain.Comput Biol Chem. 2020 Dec;89:107382. doi: 10.1016/j.compbiolchem.2020.107382. Epub 2020 Sep 24. Comput Biol Chem. 2020. PMID: 33010785
-
Oral manifestations, dental management, and a rare homozygous mutation of the PRDM12 gene in a boy with hereditary sensory and autonomic neuropathy type VIII: a case report and review of the literature.J Med Case Rep. 2017 Aug 15;11(1):233. doi: 10.1186/s13256-017-1387-z. J Med Case Rep. 2017. PMID: 28807049 Free PMC article. Review.
-
Midface Toddler Excoriation Syndrome (MiTES): A Review.Pediatr Dermatol. 2025 May-Jun;42(3):469-474. doi: 10.1111/pde.15917. Epub 2025 Mar 3. Pediatr Dermatol. 2025. PMID: 40033863 Review.
Cited by
-
Midface Toddler Excoriation Syndrome (MiTES) - A Case Report and Brief Review of Literature.Indian Dermatol Online J. 2025 Jun 27;16(4):684-687. doi: 10.4103/idoj.idoj_595_24. eCollection 2025 Jul-Aug. Indian Dermatol Online J. 2025. PMID: 40688148 Free PMC article. No abstract available.
References
-
- Srinivas SM, Gowda VK, Owen CM. et al. Mid-face toddler excoriation syndrome (MiTES): a new paediatric diagnosis. Clin Exp Dermatol 2017; 42:68–71. - PubMed
-
- Moss C, Srinivas SM, Sarveswaran N. et al. Midface toddler excoriation syndrome (MiTES) can be caused by autosomal recessive biallelic mutations in a gene for congenital insensitivity to pain, PRDM12. Br J Dermatol 2018; 179:1135–40. - PubMed
-
- Inamadar AC, Vinay K, Olabi B. et al. Extending the phenotype of midface toddler excoriation syndrome (MiTES): five new cases in three families with PR domain containing protein 12 (PRDM12) mutations. J Am Acad Dermatol 2019; 81:1415–17. - PubMed
-
- Noguera-Morel L, Ortiz-Cabrera NV, Campos M. et al. A case of mid-face toddler excoriation syndrome (MiTES). Pediatr Dermatol 2020; 37:345–6. - PubMed
-
- Kumar P, Das A. Midface toddler excoriation syndrome. Indian J Dermatol Venereol Leprol 2021; 87:528–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

