Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women
- PMID: 38591743
- PMCID: PMC11003221
- DOI: 10.1002/14651858.CD003376.pub4
Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women
Abstract
Background: Osteoporosis is an abnormal reduction in bone mass and bone deterioration, leading to increased fracture risk. Etidronate belongs to the bisphosphonate class of drugs which act to inhibit bone resorption by interfering with the activity of osteoclasts - bone cells that break down bone tissue. This is an update of a Cochrane review first published in 2008. For clinical relevance, we investigated etidronate's effects on postmenopausal women stratified by fracture risk (low versus high).
Objectives: To assess the benefits and harms of intermittent/cyclic etidronate in the primary and secondary prevention of osteoporotic fractures in postmenopausal women at lower and higher risk of fracture, respectively.
Search methods: We searched the Cochrane Central Register of Control Trials (CENTRAL), MEDLINE, Embase, two clinical trial registers, the websites of drug approval agencies, and the bibliographies of relevant systematic reviews. We identified eligible trials published between 1966 and February 2023.
Selection criteria: We included randomized controlled trials that assessed the benefits and harms of etidronate in the prevention of fractures for postmenopausal women. Women in the experimental arms must have received at least one year of etidronate, with or without other anti-osteoporotic drugs and concurrent calcium/vitamin D. Eligible comparators were placebo (i.e. no treatment; or calcium, vitamin D, or both) or another anti-osteoporotic drug. Major outcomes were clinical vertebral, non-vertebral, hip, and wrist fractures, withdrawals due to adverse events, and serious adverse events. We classified a study as secondary prevention if its population fulfilled one or more of the following hierarchical criteria: a diagnosis of osteoporosis, a history of vertebral fractures, a low bone mineral density T-score (≤ -2.5), or aged 75 years or older. If none of these criteria were met, we considered the study to be primary prevention.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. The review has three main comparisons: (1) etidronate 400 mg/day versus placebo; (2) etidronate 200 mg/day versus placebo; (3) etidronate at any dosage versus another anti-osteoporotic agent. We stratified the analyses for each comparison into primary and secondary prevention studies. For major outcomes in the placebo-controlled studies of etidronate 400 mg/day, we followed our original review by defining a greater than 15% relative change as clinically important. For all outcomes of interest, we extracted outcome measurements at the longest time point in the study.
Main results: Thirty studies met the review's eligibility criteria. Of these, 26 studies, with a total of 2770 women, reported data that we could extract and quantitatively synthesize. There were nine primary and 17 secondary prevention studies. We had concerns about at least one risk of bias domain in each study. None of the studies described appropriate methods for allocation concealment, although 27% described adequate methods of random sequence generation. We judged that only 8% of the studies avoided performance bias, and provided adequate descriptions of appropriate blinding methods. One-quarter of studies that reported efficacy outcomes were at high risk of attrition bias, whilst 23% of studies reporting safety outcomes were at high risk in this domain. The 30 included studies compared (1) etidronate 400 mg/day to placebo (13 studies: nine primary and four secondary prevention); (2) etidronate 200 mg/day to placebo (three studies, all secondary prevention); or (3) etidronate (both dosing regimens) to another anti-osteoporotic agent (14 studies: one primary and 13 secondary prevention). We discuss only the etidronate 400 mg/day versus placebo comparison here. For primary prevention, we collected moderate- to very low-certainty evidence from nine studies (one to four years in length) including 740 postmenopausal women at lower risk of fractures. Compared to placebo, etidronate 400 mg/day probably results in little to no difference in non-vertebral fractures (risk ratio (RR) 0.56, 95% confidence interval (CI) 0.20 to 1.61); absolute risk reduction (ARR) 4.8% fewer, 95% CI 8.9% fewer to 6.1% more) and serious adverse events (RR 0.90, 95% CI 0.52 to 1.54; ARR 1.1% fewer, 95% CI 4.9% fewer to 5.3% more), based on moderate-certainty evidence. Etidronate 400 mg/day may result in little to no difference in clinical vertebral fractures (RR 3.03, 95% CI 0.32 to 28.44; ARR 0.02% more, 95% CI 0% fewer to 0% more) and withdrawals due to adverse events (RR 1.41, 95% CI 0.81 to 2.47; ARR 2.3% more, 95% CI 1.1% fewer to 8.4% more), based on low-certainty evidence. We do not know the effect of etidronate on hip fractures because the evidence is very uncertain (RR not estimable based on very low-certainty evidence). Wrist fractures were not reported in the included studies. For secondary prevention, four studies (two to four years in length) including 667 postmenopausal women at higher risk of fractures provided the evidence. Compared to placebo, etidronate 400 mg/day may make little or no difference to non-vertebral fractures (RR 1.07, 95% CI 0.72 to 1.58; ARR 0.9% more, 95% CI 3.8% fewer to 8.1% more), based on low-certainty evidence. The evidence is very uncertain about etidronate's effects on hip fractures (RR 0.93, 95% CI 0.17 to 5.19; ARR 0.0% fewer, 95% CI 1.2% fewer to 6.3% more), wrist fractures (RR 0.90, 95% CI 0.13 to 6.04; ARR 0.0% fewer, 95% CI 2.5% fewer to 15.9% more), withdrawals due to adverse events (RR 1.09, 95% CI 0.54 to 2.18; ARR 0.4% more, 95% CI 1.9% fewer to 4.9% more), and serious adverse events (RR not estimable), compared to placebo. Clinical vertebral fractures were not reported in the included studies.
Authors' conclusions: This update echoes the key findings of our previous review that etidronate probably makes or may make little to no difference to vertebral and non-vertebral fractures for both primary and secondary prevention.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
None at present.
This review was updated without the support of any industry sponsor.
Figures
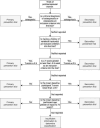
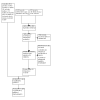

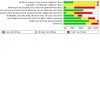












































































Update of
-
Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women.Cochrane Database Syst Rev. 2008 Jan 23;2008(1):CD003376. doi: 10.1002/14651858.CD003376.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2024 Apr 9;4:CD003376. doi: 10.1002/14651858.CD003376.pub4. PMID: 18254018 Free PMC article. Updated.
References
References to studies included in this review
Adami 2000 {published data only}
-
- Adami S, Bruni V, Bianchini D, Becorpi A, Lombardi P, Campagnoli C, et al. Prevention of early postmenopausal bone loss with cyclical etidronate. Journal of Endocrinological Investigation 2000;23(5):310-6. - PubMed
Chilibeck 2002 {published data only}
-
- Chilibeck PD, Davison KS, Whiting SJ, Suzuki Y, Janzen CL, Peloso P. The effect of strength training combined with bisphosphonate (etidronate) therapy on bone mineral, lean tissue, and fat mass in postmenopausal women. Canadian Journal of Physiology and Pharmacology 2002;80(10):941-50. - PubMed
Evans 1993 {published data only}
-
- Evans RA, Somers NM, Dunstan CR, Royle H, Kos S. The effect of low-dose cyclical etidronate and calcium on bone mass in early postmenopausal women. Osteoporosis International 1993;3(2):71-5. - PubMed
Fukunaga 2002 {published data only}
-
- Fukunaga M, Kushida K, Kishimoto H, Shiraki M, Taketani Y, Minaguchi H, et al. A comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial. Osteoporosis International 2002;13:971-9. - PubMed
Guañabens 2000 {published data only}
-
- Guañabens N, Farrerons J, Perez-Edo L, Monegal A, Renau A, Carbonell J, et al. Cyclical etidronate versus sodium fluoride in established postmenopausal osteoporosis: a randomized 3 year trial. Bone 2000;27(1):123-8. - PubMed
Gürlek 1997 {published data only}
-
- Gürlek A, Bayraktar M, Gedik O. Comparison of calcitriol treatment with etidronate-calcitriol and calcitonin-calcitriol combinations in Turkish women with postmenopausal osteoporosis: a prospective study. Calcified Tissue International 1997;61(1):39-43. - PubMed
Hasling 1994 {published data only}
-
- Hasling C, Charles P, Jensen FT, Mosekilde L. A comparison of the effects of oestrogen/progestogen, high-dose oral calcium, intermittent cyclic etidronate and an ADFR regime on calcium kinetics and bone mass in postmenopausal women with spinal osteoporosis. Osteoporosis International 1994;4:191-203. - PubMed
Heath 2000 {published data only}
-
- Heath DA, Bullivant BG, Boiven C, Balena R. The effects of cyclical etidronate on early postmenopausal bone loss: an open, randomized controlled study. Journal of Clinical Densitometry 2000;3(1):27-33. - PubMed
Herd 1997 {published data only}
-
- Herd RJ, Balena R, Blake GM, Ryan PJ, Fogelman I. The prevention of early postmenopausal bone loss by cyclical etidronate therapy: a 2-year, double-blind, placebo-controlled study. American Journal of Medicine 1997;103(2):92-9. - PubMed
Hu 2005 {published data only}
-
- Hu YF, Sun ZQ. Quality of life in the treatment assessment of postmenopausal osteoporosis [生活质量指标在绝经后骨质疏松症医疗后果评价中的作用]. Journal of Central South University (Medical Sciences) 2005;30(3):299-303. - PubMed
Ishida 2004 {published data only}
-
- Ishida Y, Kawai S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. American Journal of Medicine 2004;117(8):549-55. - PubMed
Iwamoto 2001 {published data only}
-
- Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. Journal of Orthopaedic Science 2001;6(6):487-92. - PubMed
Iwamoto 2003b {published data only}
-
- Iwamoto J, Takeda T, Ichimura S, Matsu K, Uzawa M. Effects of cyclical etidronate with alfacalcidol on lumbar bone mineral density, bone resorption, and back pain in postmenopausal women with osteoporosis. Journal of Orthopaedic Science 2003;8(4):532-7. - PubMed
Iwamoto 2005 {published data only}
Köşüş 2005 {published data only}
-
- Köşüş A, Capar M, Köşüş N. Cyclical alendronate treatment in postmenopausal women with osteoporosis. International Journal of Gynecology & Obstetrics 2005;91(2):182-4. - PubMed
Lyritis 1997 {published data only}
-
- Lyritis GP, Tsakalakos N, Paspati I, Skarantavos G, Galanos A, Androulakis C. The effect of a modified etidronate cyclical regimen on postmenopausal osteoporosis: a four-year study. Clinical Rheumatology 1997;16(4):354-60. - PubMed
Masud 1998 {published data only}
-
- Masud T, Mulcahy B, Thompson AV, Donnelly S, Keen RW, Doyle DV, et al. Effects of cyclical etidronate combined with calcitriol versus cyclical etidronate alone on spine and femoral neck bone mineral density in postmenopausal osteoporotic women. Annals of the Rheumatic Diseases 1998;57(6):346-9. - PMC - PubMed
Meunier 1997 {published data only}
-
- Meunier PJ, Confavreux E, Tupinon I, Hardouin C, Delmas PD, Balena R. Prevention of early postmenopausal bone loss with cyclical etidronate therapy (a double-blind, placebo-controlled study and 1-year follow-up). Journal of Clinical Endocrinology and Metabolism 1997;82(9):2784-9. Erratum in: Journal of Clinical Endocrinology and Metabolism 1997; 82(11):3740. - PubMed
Montessori 1997 {published data only}
-
- Montessori ML, Scheele WH, Netelenbos JC, Kerkhoff JF, Bakker K. The use of etidronate and calcium versus calcium alone in the treatment of postmenopausal osteopenia: results of three years of treatment. Osteoporosis International 1997;7(1):52-8. - PubMed
Pacifici 1988 {published data only}
-
- Pacifici R, McMurtry C, Vered I, Rupich R, Avioli LV. Coherence therapy does not prevent axial bone loss in osteoporotic women: a preliminary comparative study. Journal of Clinical Endocrinology and Metabolism 1988;66(4):747-53. - PubMed
Pouilles 1997 {published data only}
-
- Pouilles JM, Tremollieres F, Roux C, Sebert JL, Alexandre C, Goldberg D, et al. Effects of cyclical etidronate therapy on bone loss in early postmenopausal women who are not undergoing hormonal replacement therapy. Osteoporosis International 1997;7(3):213-8. - PubMed
Russo 1996 {published data only}
-
- Russo MS, Panebianco P, Stefano F, Scarpinato RA, Destro G, Salamone SA, et al. The use of bisphosphonates in the treatment of osteoporosis. Archives of Gerontology and Geriatrics 1996;22(Suppl 1):551-5. - PubMed
Sahota 2000 {published data only}
-
- Sahota O, Fowler I, Blackwell PJ, Lawson N, Cawte SA, San P, et al. A comparison of continuous alendronate, cyclical alendronate and cyclical etidronate with calcitriol in the treatment of postmenopausal vertebral osteoporosis: a randomized controlled trial. Osteoporosis International 2000;11:959-66. - PubMed
Shiota 2001 {published data only}
-
- Shiota E, Tsuchiya K, Yamaoka K, Kawano O. Effect of intermittent cyclical treatment with etidronate disodium (HEBP) and calcium plus alphacalcidol in postmenopausal osteoporosis. Journal of Orthopaedic Science 2001;6(2):133-6. - PubMed
Steiniche 1991 {published data only}
-
- Steiniche T, Hasling C, Charles P, Eriksen EF, Melsen F, Mosekilde L. The effects of etidronate on trabecular bone remodeling in postmenopausal spinal osteoporosis: a randomized study comparing intermittent treatment and an ADFR regime. Bone 1991;12(3):155-63. - PubMed
Storm 1990 {published data only}
-
- Storm T, Thamsborg G, Steiniche T, Genant HK, Sorensen OH. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. New England Journal of Medicine 1990;322(18):1265-71. - PubMed
Tobias 1997 {published data only}
-
- Tobias JH, Dalzell N, Pazianas M, Chambers TJ. Cyclical etidronate prevents spinal bone loss in early post-menopausal women. British Journal of Rheumatology 1997;36(5):612-3. - PubMed
Watts 1990 {published data only}
-
- Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P, et al. Four-year study of intermittent cyclic etidronate treatment of postmenopausal osteoporosis: three years of blinded therapy followed by one year of open therapy. American Journal of Medicine 1993;95(6):557-67. - PubMed
-
- Miller PD, Watts NB, Licata AA, Harris ST, Genant HK, Wasnich RD, et al. Cyclical etidronate in the treatment of postmenopausal osteoporosis: efficacy and safety after seven years of treatment. American Journal of Medicine 1997;103(6):468-76. - PubMed
-
- Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. New England Journal of Medicine 1990;323(2):73-9. - PubMed
Wimalawansa 1995 {published data only}
-
- Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four-year randomized study. American Journal of Medicine 1995;99(1):36-42. - PubMed
Wimalawansa 1998 {published data only}
-
- Wimalawansa SJ. A four-year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination, in women with postmenopausal osteoporosis. American Journal of Medicine 1998;104(3):219-26. - PubMed
References to studies excluded from this review
Caffarelli 2010 {published data only}
-
- Caffarelli C, Gonnelli S, Tanzilli L, Martini G, Nuti R. Apparent bone mineral density at femoral neck in the monitoring the early effects of teriparatide. Bone 2010;47:S203-4.
Fujita 1993 {published data only}
-
- Fujita T, Orimo H, Inoue T, Kaneda K, Minoru Sakurai, Morita R, et al. Double-blind multicenter comparative study with alfacalcidol of etidronate disodium (EHDP) in involutional osteoporosis. Rinsho Hyoka (Clinical Evaluation) 1993;21:261-302.
Fujita 2009 {published data only}
-
- Fujita T, Ohue M, Fujii Y, Miyauchi A, Takagi Y. Comparison of the analgesic effects of bisphosphonates: Etidronate, alendronate and risedronate by electroalgometry utilizing the fall of skin impedance. Journal of Bone and Mineral Metabolism 2009;27:234-9. - PubMed
Heaney 1976 {published data only}
-
- Heaney RP, Saville PD. Etidronate disodium in postmenopausal osteoporosis. Clinical Pharmacology & Therapeutics 1976;20(5):593-604. - PubMed
Iwamoto 2002 {published data only}
-
- Iwamoto J, Takeda T, Ichimura S. Beneficial effect of etidronate on bone loss after cessation of exercise in postmenopausal osteoporotic women. American Journal of Physical Medicine & Rehabilitation 2002;81(6):452-7. - PubMed
Iwamoto 2003a {published data only}
-
- Iwamoto J, Takeda T, Ichimura S, Uzawa M. Early response to alendronate after treatment with etidronate in postmenopausal women with osteoporosis. Keio Journal of Medicine 2003;52:113-9. - PubMed
Kushida 2004 {published data only}
-
- Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A, Inoue T, et al. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. Journal of Bone and Mineral Metabolism 2004;22:469-78. - PubMed
Miller 1999 {published data only}
-
- Miller JP, Brown ES, Siris MS, Hoseyni DW, Bekker PJ. A randomized, double-blind comparison of risedronate and etidronate in the treatment of Paget’s disease of bone. Paget’s Risedronate/Etidronate Study Group. American Journal of Medicine 1999;106:513–20. - PubMed
Pearson 1997 {published data only}
-
- Pearson EG, Nance PW, Leslie WD, Ludwig S. Cyclical etidronate: Its effect on bone density in patients with acute spinal cord injury. Archives of Physical Medicine and Rehabilitation 1997;78:269-72. - PubMed
Struijs 1996 {published data only}
-
- Struijs A, Mulder H. Treatment of post-menopausal osteoporosis and low serum magnesium with intermittent cyclical EHDP and magnesium. Osteoporosis International 1996;6(Suppl 1):252.
Yamaguchi 2005 {published data only}
-
- Yamaguchi K, Masuhara K, Yamasaki S, Fuji T. Efficacy of different dosing schedules of etidronate for stress shielding after cementless total hip arthroplasty. Journal of Orthopaedic Science 2005;10:32-6. - PubMed
Zhu 2004 {published data only}
-
- Zhu XY, Zhang YQ, Pei LC, Li X, Yu WG. Effects of combined treatment of rocaltrol, etidronate and sisterly on bone pain and bone mineral density in osteoporosis patients with vertebral fracture. Chinese Journal of Clinical Rehabilitation 2004;8:5182-3.
References to studies awaiting assessment
Clemente 1996 {published data only}
-
- Clemente PA, Cardama F, Armengol R, Gomà MT. Effect of therapeutical association between hormone replacement therapy and cyclic intermittent therapy with etidronate about bone mineral content in women with postmenopausal osteoporosis. Progresos de Obstetricia y Ginecología 1996;39(8):593-7.
Dogan 2001 {published data only}
-
- Dogan B, Guzel R, Adam M, Sarpel T, Goncu MK. Comparison of different therapeutic regimens in the treatment of patients with post menopausal osteoporosis. Journal of Rheumatology and Medical Rehabilitation 2001;12(3):143-7.
JapicCTI‐050093 {published data only}
-
- JapicCTI-050093. A comparison of incidences of vertebral fracture in Japanese patients with osteoporosis treated with etidronate and alfacalcidol; a randomized, double-blind study. rctportal.niph.go.jp/s/detail/um?trial_id=JapicCTI-050093# (first received 13 September 2005).
Lozano‐Tonkin 1996 {published data only}
-
- Lozano-Tonkin C, González MA, Garcia L, Jareňo J. Long-term treatment of postmenopausal osteoporosis with sodium etidronate or calcitonin [abstract]. Osteoporosis International 1996;6(Suppl 1):254.
Nozaki 2002 {published data only}
-
- Nozaki M, Koera K, Egami R, Nagata H, Nakano H. Combination of intermittent cyclical etidronate and hormone replacement therapy for postmenopausal non-responders to estrogen. Clinical Drug Investigation 2002;22:111-7. - PubMed
Additional references
Avenell 2020
-
- Avenell A. An influential osteoporosis study is “likely fraudulent”- but not retracted. retractionwatch.com/2020/07/02/an-influential-osteoporosis-study-is-like... (accessed 5 October 2020).
Barrionuevo 2019
-
- Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network met-analysis. Journal of Clinical Endocrinology and Metabolism 2019;104(5):1623-30. - PubMed
Black 2001
-
- Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, Hoseyni MS, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporosis International 2001;12(7):519-28. - PubMed
Brockhaus 2014
-
- Brockhaus AC, Bender R, Skipka G. The Peto odds ratio viewed as a new effect measure. Statistics in Medicine 2014;33:4861-74. - PubMed
Brown 2002
Cauley 2000
-
- Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporosis International 2000;11(7):556-61. - PubMed
Chandran 2019
Crandall 2014
-
- Crandall CJ, Newberry SJ, Diamant A, Lim YW, Gellad WF, Booth MJ, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Annals of Internal Medicine 2014;161(10):711-23. - PubMed
Cranney 2001
-
- Cranney A, Guyatt G, Krolicki N, Welch V, Griffith L, Adachi JD, et al. A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporosis International 2001;12(2):140-51. - PubMed
Cummings 1989
-
- Cummings SR, Black DM, Rubin SM. Lifetime risks of hip, Colles', or vertebral fracture and coronary heart disease among white postmenopausal women. Archives of Internal Medicine 1989;149(11):2445-8. - PubMed
Deeks 2022
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
DistillerSR [Computer program]
-
- DistillerSR. Version 2.35. DistillerSR Inc, 2023. Available at: www.distillersr.com/.
Dixon 2003a
-
- Dixon P. The p-value fallacy and how to avoid it. Canadian Journal of Experimental Psychology 2003;57(3):189-202. - PubMed
Doherty 2001
-
- Doherty DA, Sanders KM, Kotowicz MA, Prince RL. Lifetime and five-year age-specific risks of first and subsequent osteoporotic fractures in postmenopausal women. Osteoporosis International 2001;12(1):16-23. - PubMed
Edwards 2013
-
- Edwards BJ, Bunta AD, Lane J, Odvina C, Rao DS, Raisch DW, et al. Bisphosphonates and nonhealing femoral fractures: analysis of the FDA Adverse Event Reporting System (FAERS) and international safety efforts: a systematic review from the Research on Adverse Drug Events And Reports (RADAR) project. Journal of Bone and Joint Surgery 2013;95(4):297-307. - PMC - PubMed
Ellis 2014
-
- Ellis AG, Reginster JY, Luo X, Cappelleri JC, Chines A, Sutradhar S, et al. Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis. Value in Health 2014;17(4):424-32. [OTHER] - PMC - PubMed
Fink 2005
-
- Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? Journal of Bone and Mineral Research 2005;20(7):1216-22. - PubMed
Fleisch 1997
-
- Fleisch HA. Bisphosphonates: preclinical aspects and use in osteoporosis. Annals of Medicine 1997;29(1):55-62. - PubMed
Frost 1980
-
- Frost HM. The ADFR concept and monitoring it. Metabolic Bone Disease and Related Research 1980;2(Suppl):317–21.
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed prior to 20 November 2023. McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Greenspan 2001
-
- Greenspan S L, Stetten E, Emond S K, Jones L, Parker R A. Instant vertebral assessment: a noninvasive dual X-ray absorptiometry technique to avoid misclassification and clinical mismanagement of osteoporosis. Journal of Clinical Densitometry 2001;4(4):373-80. - PubMed
Hewitt 2005
-
- Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral bloodgdT cells in response to aminobisphosphonates is inhibited by statins. Clinical and Experimental Immunology 2005;139:101-11. - PMC - PubMed
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
-
- Higgins JP, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological Expectations of Cochrane Intervention Reviews (MECIR); February 2022. Available at: community.cochrane.org/sites/default/files/uploads/MECIR%20Version%20Feb... Accessed prior to 17 March 2024.
Higgins 2023
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Hodsman 2002
Hopkins 2011
Ioachimescu 2007
-
- Ioachimescu A, Licata A. Etidronate: what is its place in treatment of primary osteoporosis and other demineralizing diseases today? Current Osteoporosis Reports 2007;5(4):165-9. - PubMed
Jansen 2011
-
- Jansen JP, Bergman GJ, Huels J, Olson M. The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Seminars in Arthritis and Rheumatism 2011;40(4):275-84. - PubMed
Johnell 2006
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis International 2006;17:1726-33. - PubMed
Kanis 2013
Khow 2017
-
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. Journal of Nutrition, Health and Aging 2017;21(1):83-91. - PubMed
Koziol 2017
-
- Koziol M. A shadow was cast on a bone researcher’s work. What are journals doing about his papers? retractionwatch.com/author/michael-koziol/ (accessed 5 October 2020).
Langsetmo 2009
Lee 2014
-
- Lee SH, Chang SS, Lee M, Chan RC, Lee CC. Risk of osteonecrosis in patients taking bisphosphonates for prevention of osteoporosis: a systematic review and meta-analysis. Osteoporosis International 2014;25(3):1131-9. - PubMed
Lefebvre 2022
-
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Liu 2018
-
- Liu GF, Wang ZQ, Liu L, Zhang BT, Miao YY, Yu SN. A network meta-analysis on the short-term efficacy and adverse events of different anti-osteoporosis for the treatment of postmenopausal osteoporosis. Journal of Cell Biochemistry 2018;119(6):4469-81. - PubMed
Mavrokokki 2007
-
- Mavrokokki T, Cheng A, Stein B, Gross A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. Journal of Oral Maxillofacial Surgery 2007;65:415-23. - PubMed
McGowan 2016
-
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. Journal of Clinical Epidemiology 2016;75:40-6. - PubMed
Meier 2012a
-
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Archives of Internal Medicine 2012;172(12):930-6. - PubMed
Melton 2013
Migliore 2013
-
- Migliore A, Broccoli S, Massafra U, Cassol M, Frediani B. Ranking antireabsorptive agents to prevent vertebral fractures in postmenopausal osteoporosis by mixed treatment comparison meta-analysis. European Review for Medical and Pharmacological Sciences 2013;17(5):658-67. - PubMed
Newell 1992a
-
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837-41. - PubMed
NIH Consensus 2001
-
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285(6):785-95. - PubMed
NOGG 2017
-
- National Osteoporosis Guideline Group. NOGG 2017: Clinical guideline for the prevention and treatment of osteoporosis. National Osteoporosis Guideline Group Updated March 2017.
Papaioannou 2010
Pasco 2006
-
- Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporosis International 2006;17(9):1404-9. - PubMed
Rohatgi 2019 [Computer program]
-
- WebPlotDigitizer Version: 4.2. Rohatgi A. Austin, Texas: San Francisco, California, April 2019. Available at: automeris.io/WebPlotDigitizer.
Rosen 1997
-
- Rosen CJ. A tale of two worlds in prescribing etidronate for osteoporosis. Lancet 1997;350:1340. - PubMed
Santesso 2020
-
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guideline 26: informative statements communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. - PubMed
Schünemann 2022a
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sharma 2014
-
- Sharma A, Einstein AJ, Vallakati A, Arbab-Zadeh A, Walker MD, et al. Risk of atrial fibrillation with use of oral and intravenous bisphosphonates. American Journal of Cardiology 2014;113(11):1815-21. - PubMed
Tadrous 2014
-
- Tadrous M, Wong L, Mamdani MM, Juurlink DN, Krahn MD, Levesque LE, et al. Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: a network meta-analysis. Osteoporosis International 2014;25(4):1225-35. - PubMed
Taggart 2002
-
- Taggart H, Bolognese MA, Lindsay R, Ettinger MP, Mulder H, Josse RG, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clinic Proceedings 2002;77(3):262-70. - PubMed
Tran 2017a
-
- Tran T, Bliuc D, Geel T, Adachi JD, Berger C, den Bergh J, et al. Population-Wide Impact of Non-Hip Non-Vertebral Fractures on Mortality. Journal of Bone and Mineral Research 2017;32(9):1802-10. - PubMed
Wade 2014a
-
- Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Archives of Osteoporosis 2014;9:1-10. - PubMed
Wainwright 2005a
-
- Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, et al. Hip fracture in women withoutosteoporosis. Journal of Clinical Endocrinology and Metabolism 2005;90(5):2787-93. - PubMed
WHO 1994
-
- World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. World Health Organization Technical Report Series 1994;843:1-129. - PubMed
WHO 2004
-
- World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level: summary meeting report; 2004. Available at www.who.int/chp/topics/Osteoporosis.pdf (accessed 6 February 2020).
Yang 2016a
-
- Yang XC, Deng ZH, Wen T, Luo W, Xiao WF, Zhao RB, et al. Network meta-analysis of pharmacological agents for osteoporosis treatment and fracture prevention. Cellular Physiology and Biochemistry 2016;40(3-4):781-95. - PubMed
Zhou 2016
-
- Zhou J, Ma X, Wang T, Zhai S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network meta-analyses. Osteoporosis International 2016;27(11):3289-300. - PubMed
References to other published versions of this review
Wells 2006b
-
- Wells GA, Cranney A, Bouchere M, Peterson J, Shea B, Robinson V, et al. Bisphosphonates for the primary and secondary prevention of osteoporotic fractures in postmenopausal women: a meta-analysis [Technology report no. 69]. Ottawa: Canadian Agency for Drugs and Technologies in Health 2006.
Wells 2008a
-
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Welch V, et al. Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women (Review). Cochrane Database of Systematic Reviews 2008, Issue 7. Art. No: CD003376. [DOI: 10.1002/14651858.CD003376.pub3] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

