Current perspectives on the multiple roles of osteoclasts: Mechanisms of osteoclast-osteoblast communication and potential clinical implications
- PMID: 38591777
- PMCID: PMC11003748
- DOI: 10.7554/eLife.95083
Current perspectives on the multiple roles of osteoclasts: Mechanisms of osteoclast-osteoblast communication and potential clinical implications
Abstract
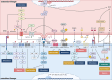
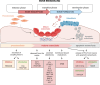
Bone remodeling is a complex process involving the coordinated actions of osteoblasts and osteoclasts to maintain bone homeostasis. While the influence of osteoblasts on osteoclast differentiation is well established, the reciprocal regulation of osteoblasts by osteoclasts has long remained enigmatic. In the past few years, a fascinating new role for osteoclasts has been unveiled in promoting bone formation and facilitating osteoblast migration to the remodeling sites through a number of different mechanisms, including the release of factors from the bone matrix following bone resorption and direct cell-cell interactions. Additionally, considerable evidence has shown that osteoclasts can secrete coupling factors known as clastokines, emphasizing the crucial role of these cells in maintaining bone homeostasis. Due to their osteoprotective function, clastokines hold great promise as potential therapeutic targets for bone diseases. However, despite long-standing work to uncover new clastokines and their effect in vivo, more substantial efforts are still required to decipher the mechanisms and pathways behind their activity in order to translate them into therapies. This comprehensive review provides insights into our evolving understanding of the osteoclast function, highlights the significance of clastokines in bone remodeling, and explores their potential as treatments for bone diseases suggesting future directions for the field.
Keywords: bone homeostasis; clastokines; communication; coupling; medicine; osteoblasts; osteoclasts.
Conflict of interest statement
VD, KH, HD No competing interests declared
Figures

Similar articles
-
The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications.Front Immunol. 2022 Apr 26;13:869422. doi: 10.3389/fimmu.2022.869422. eCollection 2022. Front Immunol. 2022. PMID: 35558080 Free PMC article. Review.
-
Osteoclast Secretes Stage-Specific Key Molecules for Modulating Osteoclast-Osteoblast Communication.J Cell Physiol. 2025 Jan;240(1):e31484. doi: 10.1002/jcp.31484. Epub 2024 Nov 28. J Cell Physiol. 2025. PMID: 39606839 Review.
-
Osteoblast-osteoclast interactions.Connect Tissue Res. 2018 Mar;59(2):99-107. doi: 10.1080/03008207.2017.1290085. Epub 2017 Mar 21. Connect Tissue Res. 2018. PMID: 28324674 Free PMC article. Review.
-
Osteoblast and osteoclast crosstalks: from OAF to Ephrin.Inflamm Allergy Drug Targets. 2012 Jun;11(3):196-200. doi: 10.2174/187152812800392670. Inflamm Allergy Drug Targets. 2012. PMID: 22280242 Review.
-
Maintenance of Bone Homeostasis by DLL1-Mediated Notch Signaling.J Cell Physiol. 2017 Sep;232(9):2569-2580. doi: 10.1002/jcp.25647. Epub 2017 Mar 31. J Cell Physiol. 2017. PMID: 27735989 Free PMC article.
Cited by
-
Osteoclast-Driven Polydopamine-to-Dopamine Release: An Upgrade Patch for Polydopamine-Functionalized Tissue Engineering Scaffolds.J Funct Biomater. 2024 Jul 29;15(8):211. doi: 10.3390/jfb15080211. J Funct Biomater. 2024. PMID: 39194649 Free PMC article.
-
Hyperbaric oxygen potentiates platelet-rich plasma composition and accelerates bone healing.J Orthop Translat. 2025 Jan 19;51:1-12. doi: 10.1016/j.jot.2024.10.016. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 39902098 Free PMC article.
-
Multifunctional Injectable Bioadhesive with Toll-like Receptor 4 and Myeloid Differentiation Factor 2 Antagonistic Anti-inflammatory Potential for Periodontal Regeneration.ACS Nano. 2025 Feb 25;19(7):7098-7116. doi: 10.1021/acsnano.4c15922. Epub 2025 Feb 14. ACS Nano. 2025. PMID: 39951685 Free PMC article.
-
Biodegradable Zn-0.8Mg-0.2Sr alloy as an internal fixation material exhibits controlled degradation with enhanced osteogenesis.RSC Adv. 2025 Aug 22;15(37):30071-30088. doi: 10.1039/d5ra02009c. eCollection 2025 Aug 22. RSC Adv. 2025. PMID: 40860423 Free PMC article.
-
Chronic heavy alcohol consumption impairs the ability of demineralized allogenic bone matrix to support osteoinduction in alcohol-naïve rats.Bone Rep. 2025 Mar 12;25:101836. doi: 10.1016/j.bonr.2025.101836. eCollection 2025 Jun. Bone Rep. 2025. PMID: 40171448 Free PMC article.
References
-
- Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, Schuster B, Kallen KJ, Saftig P, Rose-John S, Ludwig A. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. Journal of Immunology. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. - DOI - PubMed
-
- Aoki K, Saito H, Itzstein C, Ishiguro M, Shibata T, Blanque R, Mian AH, Takahashi M, Suzuki Y, Yoshimatsu M, Yamaguchi A, Deprez P, Mollat P, Murali R, Ohya K, Horne WC, Baron R. A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. The Journal of Clinical Investigation. 2006;116:1525–1534. doi: 10.1172/JCI22513. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

