Longer and better lives for patients with atrial fibrillation: the 9th AFNET/EHRA consensus conference
- PMID: 38591838
- PMCID: PMC11003300
- DOI: 10.1093/europace/euae070
Longer and better lives for patients with atrial fibrillation: the 9th AFNET/EHRA consensus conference
Abstract
Aims: Recent trial data demonstrate beneficial effects of active rhythm management in patients with atrial fibrillation (AF) and support the concept that a low arrhythmia burden is associated with a low risk of AF-related complications. The aim of this document is to summarize the key outcomes of the 9th AFNET/EHRA Consensus Conference of the Atrial Fibrillation NETwork (AFNET) and the European Heart Rhythm Association (EHRA).
Methods and results: Eighty-three international experts met in Münster for 2 days in September 2023. Key findings are as follows: (i) Active rhythm management should be part of the default initial treatment for all suitable patients with AF. (ii) Patients with device-detected AF have a low burden of AF and a low risk of stroke. Anticoagulation prevents some strokes and also increases major but non-lethal bleeding. (iii) More research is needed to improve stroke risk prediction in patients with AF, especially in those with a low AF burden. Biomolecules, genetics, and imaging can support this. (iv) The presence of AF should trigger systematic workup and comprehensive treatment of concomitant cardiovascular conditions. (v) Machine learning algorithms have been used to improve detection or likely development of AF. Cooperation between clinicians and data scientists is needed to leverage the potential of data science applications for patients with AF.
Conclusions: Patients with AF and a low arrhythmia burden have a lower risk of stroke and other cardiovascular events than those with a high arrhythmia burden. Combining active rhythm control, anticoagulation, rate control, and therapy of concomitant cardiovascular conditions can improve the lives of patients with AF.
Keywords: AFNET; Anticoagulation; Artificial intelligence; Atrial cardiomyopathy; Atrial fibrillation; Biomarkers; Bleeding; Catheter ablation; Cognitive function; Consensus statement; Cost; Dementia; EHRA; Guidelines; Heart failure; Integrated care; Outcomes; Quality of care; Research; Research priorities; Rhythm management; Screening; Stroke; Technology.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest The 9th AFNET/EHRA consensus conference was partially supported by the European Union MAESTRIA project (grant agreement 965286) to AFNET. The following participants and authors are employees of companies active in cardiovascular health as indicated in their affiliations: M.D.M., E.D., C.E., G.H., L.H.H., T.H., R.H.v.L., M.W., and H.W. P.K. was partially supported by the European Union AFFECT-AF (grant agreement 847770) and MAESTRIA (grant agreement 965286), German Center for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK, grant numbers DZHK FKZ 81X2800182, 81Z0710116, and 81Z0710110), German Research Foundation (Ki 509167694), and Leducq Foundation. He receives research support for basic, translational, and clinical research projects from several drug and device companies active in AF and has received honoraria from several such companies in the past, but not in the last 3 years. He is listed as an inventor on two issued patents held by the University of Hamburg (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). J.G.A. was partially supported by the Canadian Arrhythmia Network and the Michael Smith Foundation for Health Research, Baylis Medical. He receives consulting fees/honoraria from Bayer, BMS/Pfizer Alliance, Servier, and Medtronic Inc. E.A. receives consulting fees/honoraria from Biosense Webster and Bayer. G.B. receives consulting fees/honoraria from Bayer, BMS, Boston Scientific, Daiichi Sankyo, Sanofi, and Janssen. A.J.C. receives consulting fees/honoraria from Bayer, Pfizer/BMS, Daiichi Sankyo, Menarini, Sanofi, Boston Scientific, Biosense Webster, Abbott, Acesion Pharma, Huya Bio, and Milestone. V.C. receives consulting fees/honoraria from Bayer, Boehringer Ingelheim, and Ever Pharma (paid to the institution of employment). W.D. receives consulting fees/honoraria from Reata and research grants from MicroPort, Boston Scientific, and Abbott. S.Z.D. receives consulting fees from BMS/Pfizer, Cortrium, and Acesion Pharma and speaker fees from MS/Pfizer and Bayer. He is listed as a medical advisor for Vital Beats. Dobromir D. receives consulting fees/honoraria from Elsevier, Springer Healthcare Ltd, and Daiichi Sankyo and research grants as follows: four NIH grants (partially) from Baylor College of Medicine, Houston; one NIH grant from UC Davis, one NIH grant from the University of Minnesota, and one EU-Project H2020. David D. receives consulting fees/honoraria from Abbott, Astra Zeneca, Biotronik, Boehringer Ingelheim, Boston Scientific, BMS/Pfizer, CVRx, Medtronic, MicroPort, and Zoll and research grants from Roche, CVRx, and Zoll. L.E. has received lecture fees from various companies in AF in the past but none related to the present work. L.F. receives consulting fees/honoraria from Roche (paid to the institution of employment). She is currently employed at the UKE and previously at the University of Birmingham. She was partially supported by the European Union AFFECT-EU (grant agreement 847770), MAESTRIA (grant agreement 965286), CATCH ME (grant agreement 633196), and the British Heart Foundation (AA/18/2/3218). D.F.-R. receives research grants from Abbott. He is listed as an inventor on two issued patents: EP3636147A1 (method for the identification of cardiac fibrillation drivers and/or the footprint of rotational activations) and PCT/EP2022/071364 (system and method of assessment of electromechanical remodelling). A.G. receives consulting fees/honoraria from Daiichi Sankyo, Bayer, BMS/Pfizer, Medtronic, Abbott, and Boston Scientific and was partially supported by the European Union MAESTRIA (grant agreement 965286). K.G.H. receives consulting fees/honoraria from Abbott, Alexion, Amarin, Astra Zeneca, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, BMS/Pfizer, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novaris, Portola, Premier Research, Sanofi, SUN Pharma, and W. L. Gore and Associates. J.S.H. receives speaking fees from BMS/Pfizer, Bayer, Servier, and Boston Scientific and consulting fees from Bayer and Boston Scientific. He receives research grants from BMS/Pfizer, Servier, Novartis, Boston Scientific, and Medtronic. H.H. receives lecture and consulting fees from Bayer, Biotronik, BMS/Pfizer, Daiichi Sankyo, Milestone Pharmaceuticals, Centrix India, C.T.I. Germany, ESC, Medscape, and Springer Healthcare Ltd. He receives research grants (paid to the institution of employment, University of Antwerp and/or University of Hasselt) from Abbott, Bayer, Biosense Webster, Boston Scientific, Daiichi Sankyo, Fibricheck/Qompium, Medtronic, and BMS/Pfizer. Z.H. receives consulting fees/honoraria from Boehringer Ingelheim, BMS/Pfizer, and Roche Diagnostics. He was partially supported by The Swedish Society for Medical Research (S17-0133), Hjärt-Lungfonden (The Swedish Heart-Lung Foundation, 20200722), and the institution he is currently employed at (Uppsala University Hospital). L.H.-M. receives research grants from the Spanish Ministry of Science and Innovation (PID2020-116927RB-C21) and Fondo Europeo de Desarrollo Regional (FEDER). D.K. receives consulting fees/honoraria from Bayer, Amomed, and Protherics Medicines Development. He receives research grants from the National Institute for Health Research (NIHR CDF-2015-08-074 RAE-AF; NIHR130280 DaRe2THINK; NIHR13274 D2T-NeuroVascular; and NIHR203326 Biomedical Research Centre), the British Heart Foundation (PG/17/55/33087, AA/182/3218, and FS/CDRF/21/21032), the EU/EFPIA Innovative Medicines Initiative (BigData@Heart 116074), EU Horizon and UKRI (HYPERMARKER 101095480) UK National Health Service—Data for R&D-Subnational Secure Data Environment programme, UK Department for Business, Energy Industrial Strategy Regulators Pioneer Fund, the Cook & Wolstenholme Charitable Trust, and the European Society of Cardiology supported by educational grants from Boehringer Ingelheim, BMS/Pfizer, Alliance, Bayer, Daiichi Sankyo, Boston Scientific, the NIHR/University of Oxford Biomedical Research Centre, and the British Hear Foundation, the University of Birmingham Accelerator Award (STEEER-AF). J.L.M. receives consulting fees/honoraria from Biotronik, Medtronic, MicroPort, and Milestone Pharmaceuticals. A.M. receives consulting fees/honoraria from Medtronic, Biosense Webster, and Boston Scientific and lecture fees from Medtronic, Boston Scientific, Biosense Webster, BMS, and Bayer. L.M. receives consulting fees/honoraria from Abbott, Medtronic, Boston Scientific, and Johnson & Johnson. G.A.N. receives lecture fees from AliveCor, consultant fees from Biosense Webster, and research grants from Abbott and Biosense Webster. H.P. receives consulting fees/honoraria from Abbott, Boston Scientific, Biosense Webster, Medtronic, Daiichi Sankyo, Bayer, and Pfizer. P.S. receives consulting fees/honoraria from Medtronic, Boston Scientific, Abbott, CathRx, and PaceMate (paid to the institution of employment). He is currently employed at the University of Adelaide, which receives research grants from Medtronic, Boston Scientific, and Becton-Dickenson. R.B.S. receives consulting fees/honoraria from BMS/Pfizer. She was partially supported by the European Union Horizon 2020 research and innovation programme (grant agreement 648131 and 847770), German Center for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK, grant numbers 81Z1710103 and 81Z0710114), German Ministry of Research and Education (BMBF 01ZX1408A), ERACoSysMed3 (031L0239), Wolfgang Seefried project funding German Heart Foundation. U.S. receives consulting fees/honoraria from University Svizzerra Italiana, Stanford, and Johnson & Johnson and research grants from the European Union, Dutch Heart Foundation, Roche, and EP Solution. He is a shareholder of YourRhythmics B.V. T.T. receives consulting fees/honoraria from Boston Scientific and Medtronic. I.C.v.G. receives consulting fees/honoraria from Bayer (paid to the institution of employment). She is currently employed at the University of Groningen. K.V. receives consulting fees/honoraria from Abbott, Philips, Medtronic, Biosense Webster, and Boston Scientific and research grants from Medtronic and Biosense Webster. R.W. receives consulting fees/honoraria from Boehringer Ingelheim, BMS/Pfizer, Daiichi Sankyo, Boston Scientific, Biotronik, Abiomed, and Zoll and a research grant from Boston Scientific, BMS/Pfizer, and Abiomed. S.W. receives consulting fees/honoraria from Boehringer Ingelheim, Boston Scientific, Abbott, and Bayer Vital and a research grant from Boston Scientific. All remaining authors (G.B., J.C.N., T.D.P., N.D., M.F., E.G., S.H., S.K., D.L., K.M.-R., M.O., A.S.P., U.R., M.R., D.S., C.S., G.S., D.S., S.T., R.H.v.L., and S.Z.) have declared no conflicts of interest.
Figures


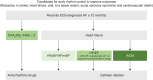
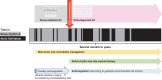

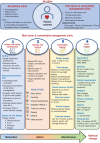

References
-
- Kirchhof P, Toennis T, Goette A, Camm AJ, Diener HC, Becher N et al. Anticoagulation with edoxaban in patients with atrial high-rate episodes. N Engl J Med 2023;389:1167–79. - PubMed
-
- Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660–71. - PubMed
-
- Sohns C, Fox H, Marrouche NF, Crijns H, Costard-Jaeckle A, Bergau L et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med 2023;389:1380–9. - PubMed
-
- Healey JS, Lopes RD, Granger CB, Alings M, Rivard L, McIntyre WF et al. Apixaban for stroke prevention in subclinical atrial fibrillation. N Engl J Med 2024;390:107–17. - PubMed
-
- Connolly S, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

