Sustained Release of Salicylic Acid for Halting Peri-Implantitis Progression in Healthy and Hyperglycemic Systemic Conditions: A Gottingen Minipig Model
- PMID: 38591966
- PMCID: PMC11094686
- DOI: 10.1021/acsbiomaterials.4c00161
Sustained Release of Salicylic Acid for Halting Peri-Implantitis Progression in Healthy and Hyperglycemic Systemic Conditions: A Gottingen Minipig Model
Abstract
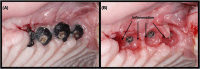
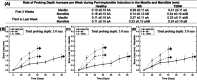
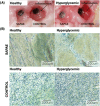
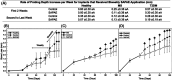
To develop a peri-implantitis model in a Gottingen minipig and evaluate the effect of local application of salicylic acid poly(anhydride-ester) (SAPAE) on peri-implantitis progression in healthy, metabolic syndrome (MS), and type-2 diabetes mellitus (T2DM) subjects. Eighteen animals were allocated to three groups: (i) control, (ii) MS (diet for obesity induction), and (iii) T2DM (diet plus streptozotocin for T2DM induction). Maxillary and mandible premolars and first molar were extracted. After 3 months of healing, four implants per side were placed in both jaws of each animal. After 2 months, peri-implantitis was induced by plaque formation using silk ligatures. SAPAE polymer was mixed with mineral oil (3.75 mg/μL) and topically applied biweekly for up to 60 days to halt peri-implantitis progression. Periodontal probing was used to assess pocket depth over time, followed by histomorphologic analysis of harvested samples. The adopted protocol resulted in the onset of peri-implantitis, with healthy minipigs taking twice as long to reach the same level of probing depth relative to MS and T2DM subjects (∼3.0 mm), irrespective of jaw. In a qualitative analysis, SAPAE therapy revealed decreased levels of inflammation in the normoglycemic, MS, and T2DM groups. SAPAE application around implants significantly reduced the progression of peri-implantitis after ∼15 days of therapy, with ∼30% lower probing depth for all systemic conditions and similar rates of probing depth increase per week between the control and SAPAE groups. MS and T2DM conditions presented a faster progression of the peri-implant pocket depth. SAPAE treatment reduced peri-implantitis progression in healthy, MS, and T2DM groups.
Keywords: dental implants; metabolic diseases; osseointegration; peri-implantitis; treatment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Bone healing around implants placed in subjects with metabolically compromised systemic conditions.J Biomed Mater Res B Appl Biomater. 2023 Sep;111(9):1664-1671. doi: 10.1002/jbm.b.35264. Epub 2023 May 15. J Biomed Mater Res B Appl Biomater. 2023. PMID: 37184298 Free PMC article.
-
Osseointegration of implant surfaces in metabolic syndrome and type-2 diabetes mellitus.J Biomed Mater Res B Appl Biomater. 2024 Feb;112(2):e35382. doi: 10.1002/jbm.b.35382. J Biomed Mater Res B Appl Biomater. 2024. PMID: 38355936 Free PMC article.
-
Evaluation of Different Implant Designs in a Ligature-Induced Peri-implantitis Model: A Canine Study.Int J Oral Maxillofac Implants. 2015 May-Jun;30(3):534-45. doi: 10.11607/jomi.3737. Int J Oral Maxillofac Implants. 2015. PMID: 26009904
-
Obesity/Metabolic Syndrome and Diabetes Mellitus on Peri-implantitis.Trends Endocrinol Metab. 2020 Aug;31(8):596-610. doi: 10.1016/j.tem.2020.05.005. Epub 2020 Jun 23. Trends Endocrinol Metab. 2020. PMID: 32591106 Review.
-
Evaluating the effect of mechanical debridement with adjunctive antimicrobial photodynamic therapy in comparison with mechanical debridement alone on the peri-implant parameters in type 2 diabetic mellitus patients with peri-implantitis: a systematic review and meta-analysis.BMC Oral Health. 2023 Oct 12;23(1):751. doi: 10.1186/s12903-023-03337-9. BMC Oral Health. 2023. PMID: 37828479 Free PMC article.
Cited by
-
An evaluation of novel AMP2-coated electrospun composite scaffolds for intraoral bone regeneration: a proof-of-concept in vivo study.Front Bioeng Biotechnol. 2025 Apr 17;13:1443280. doi: 10.3389/fbioe.2025.1443280. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40313641 Free PMC article.
References
-
- Hjalmarsson L.; Gheisarifar M.; Jemt T. A systematic review of survival of single implants as presented in longitudinal studies with a follow-up of at least 10 years. Eur. J. Oral Implantol. 2016, 9 (1), S155–S162. - PubMed
