Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms
- PMID: 38592377
- PMCID: PMC10933994
- DOI: 10.3390/jcm13051519
Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms
Abstract
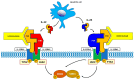
Inflammatory bowel diseases, comprising Crohn's disease (CD) and ulcerative colitis (UC), are chronic, relapsing, and remitting immune-mediated inflammatory diseases affecting the gastrointestinal tract. Ustekinumab (UST) is a monoclonal antibody that blocks the p40 subunit of the anti-interleukin (IL) 12/23. Pivotal trials (CERTIFI and UNITI-IM for CD, UNIFI for UC) established the efficacy of UST for the induction and maintenance of remission in both CD and UC, with the most favorable results in naïve patients to biologics. In recent years, a wealth of 'real-world' data has emerged supporting positive clinical, endoscopic, and histological outcomes in patients treated with UST, as well as reassuring safety data. More recently, the results of the first head-to-head trials of UST and tumor necrosis factor (TNF) antagonists were reported. Moreover, a number of studies exploring the role of UST in specific clinical settings, such as perianal CD, postoperative complications and recurrence, extraintestinal manifestations, chronic antibiotic-refractory pouchitis, and pregnancy, were reported. This review explores the results reported to date on UST, including those from pivotal trials, real-world data, and emerging studies regarding therapeutic drug monitoring and immunogenicity. The safety profile of UST was also reviewed.
Keywords: Crohn’s disease; inflammatory bowel diseases; mucosal healing; remission; safety; ulcerative colitis; ustekinumab.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Lamb C.A., Kennedy N.A., Raine T., Hendy P.A., Smith P.J., Limdi J.K., Hayee B., Lomer M.C.E., Parkes G.C., Selinger C., et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. doi: 10.1136/gutjnl-2019-318484. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources