Comparison of the efficacy of enfortumab vedotin between patients with metastatic urothelial carcinoma who were treated with avelumab or pembrolizumab: real-world data from a multi-institutional study in Japan
- PMID: 38592548
- PMCID: PMC11003883
- DOI: 10.1007/s00432-024-05717-2
Comparison of the efficacy of enfortumab vedotin between patients with metastatic urothelial carcinoma who were treated with avelumab or pembrolizumab: real-world data from a multi-institutional study in Japan
Abstract
Objectives: Enfortumab vedotin (EV) is a novel antibody-drug conjugate approved for metastatic urothelial carcinoma (UC) refractory to prior treatment with immune checkpoint inhibitors (ICIs). However, the difference in efficacy of EV after each ICIs and prognostic factors are not well known. We aimed to compare the efficacy of EV in patients with metastatic UC who were treated with avelumab or pembrolizumab and to identify the prognostic factors.
Methods: The records of 100 patients with advanced metastatic UC who received EV after the administration of either avelumab or pembrolizumab were retrospectively collected from five academic hospitals in Japan.
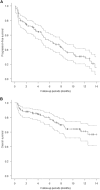
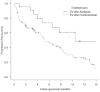
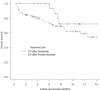
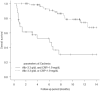
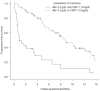
Results: The median follow-up period was 6.7 months. The median overall survival (OS) and progression-free survival (PFS) in the EV after avelumab/pembrolizumab group were not reached/14.7 months (p = 0.17) and 10.4/5.2 months (p = 0.039), respectively. The objective response rates (ORR) were 66.6% and 46.8% in EV after avelumab and EV after pembrolizumab groups, respectively (p = 0.14). Multivariate analysis identified histological variants, liver metastasis, low serum albumin levels, and high serum CRP level as significant poor prognostic factors. The median OS and PFS of cachexia patients with both low serum albumin levels and high serum CRP levels were 6.0 months and 0.93 months, respectively.
Conclusion: PFS was superior in patients treated with EV after avelumab to EV after pembrolizumab. However, OS showed no significant difference between the two groups. Because the prognosis of patients with cachexia is extremely poor, the initiation of EV should be discussed in these patients.
Keywords: Avelumab; Bladder cancer; Enfortumab vedotin; Pembrolizumab; Urothelial carcinoma.
© 2024. The Author(s).
Conflict of interest statement
All authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Enfortumab vedotin and pembrolizumab: redefining the standard of care for previously untreated advanced urothelial cancer.Future Oncol. 2025 May;21(11):1333-1348. doi: 10.1080/14796694.2025.2482363. Epub 2025 Mar 25. Future Oncol. 2025. PMID: 40129250 Free PMC article. Review.
-
Efficacy of enfortumab vedotin in advanced urothelial cancer: Analysis from the Urothelial Cancer Network to Investigate Therapeutic Experiences (UNITE) study.Cancer. 2022 Mar 15;128(6):1194-1205. doi: 10.1002/cncr.34057. Epub 2021 Dec 9. Cancer. 2022. PMID: 34882781
-
First-line systemic therapy in patients with metastatic or locally advanced urothelial carcinoma: a systematic review and network meta-analysis of randomized controlled trials.Ther Adv Med Oncol. 2025 Jul 23;17:17588359251357527. doi: 10.1177/17588359251357527. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40718544 Free PMC article.
-
Real-world clinical outcomes of sacituzumab govitecan after prior exposure to enfortumab vedotin in patients with metastatic urothelial carcinoma.ESMO Open. 2025 Jun;10(6):105305. doi: 10.1016/j.esmoop.2025.105305. Epub 2025 Jun 5. ESMO Open. 2025. PMID: 40479863 Free PMC article.
-
Enfortumab Vedotin With or Without Pembrolizumab in Metastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2025 Mar 3;8(3):e250250. doi: 10.1001/jamanetworkopen.2025.0250. JAMA Netw Open. 2025. PMID: 40067303 Free PMC article.
Cited by
-
C-Reactive Protein-Albumin Ratio Predicts Objective Response to Enfortumab Vedotin in Metastatic Urothelial Carcinoma.Target Oncol. 2024 Jul;19(4):635-644. doi: 10.1007/s11523-024-01068-7. Epub 2024 May 28. Target Oncol. 2024. PMID: 38807017
-
Clinical outcomes of avelumab and pembrolizumab in advanced urothelial cancer: an observational multicenter retro-prospective study on patients undergoing treatment in clinical practice (AVePEm study).Front Oncol. 2025 Feb 21;15:1532421. doi: 10.3389/fonc.2025.1532421. eCollection 2025. Front Oncol. 2025. PMID: 40061901 Free PMC article.
-
Cancer-induced Pain Is Associated With Poor Overall Survival of Urothelial Carcinoma Patients Treated With Enfortumab Vedotin.In Vivo. 2025 May-Jun;39(3):1533-1539. doi: 10.21873/invivo.13953. In Vivo. 2025. PMID: 40295006 Free PMC article.
-
Oncologic Outcomes of Patients with Immune Checkpoint Inhibitor Resistant Urothelial Carcinoma Treated with Enfortumab Vedotin and the Impact of Neutrophil-to-Lymphocyte Ratio and Dysgeusia on Overall Survival: A Retrospective Multicenter Cohort Study in Japan.Cancers (Basel). 2024 Jul 25;16(15):2648. doi: 10.3390/cancers16152648. Cancers (Basel). 2024. PMID: 39123376 Free PMC article.
-
Risk Classification of Patients With Advanced Urothelial Carcinoma Treated With Enfortumab Vedotin.Cancer Diagn Progn. 2024 Nov 3;4(6):783-788. doi: 10.21873/cdp.10396. eCollection 2024 Nov-Dec. Cancer Diagn Progn. 2024. PMID: 39502620 Free PMC article.
References
-
- Abuhelwa A, Bellmunt J, Kichenadasse G, McKinnon R, Rowland A, Sorich MJ et al (2022) Enhanced bellmunt risk score for survival prediction in urothelial carcinoma treated with immunotherapy. Clin Genitourin Cancer 20(2):132–138 - PubMed
-
- Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G et al (2009) Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 27:4454–4461 - PubMed
-
- Bellmunt J, Choueiri TK, Fougeray R, Schutz F, Salhi Y, Winquist E et al (2010) Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28(11):1850–1855 - PubMed
-
- Bellmunt J, Fougeray R, Rosenberg JE, von der Maase H, Schutz FA, Salhi Y et al (2013) Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol 24:1466–1472 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

