Adapting Ferritin, a Naturally Occurring Protein Cage, to Modulate Intrinsic Agonism of OX40
- PMID: 38592684
- PMCID: PMC11099885
- DOI: 10.1021/acs.bioconjchem.4c00020
Adapting Ferritin, a Naturally Occurring Protein Cage, to Modulate Intrinsic Agonism of OX40
Abstract
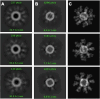
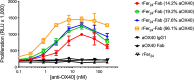
Ferritin is a multivalent, self-assembling protein scaffold found in most human cell types, in addition to being present in invertebrates, higher plants, fungi, and bacteria, that offers an attractive alternative to polymer-based drug delivery systems (DDS). In this study, the utility of the ferritin cage as a DDS was demonstrated within the context of T cell agonism for tumor killing. Members of the tumor necrosis factor receptor superfamily (TNFRSF) are attractive targets for the development of anticancer therapeutics. These receptors are endogenously activated by trimeric ligands that occur in transmembrane or soluble forms, and oligomerization and cell-surface anchoring have been shown to be essential aspects of the targeted agonism of this receptor class. Here, we demonstrated that the ferritin cage could be easily tailored for multivalent display of anti-OX40 antibody fragments on its surface and determined that these arrays are capable of pathway activation through cell-surface clustering. Together, these results confirm the utility, versatility, and developability of ferritin as a DDS.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Harrison P. M. The Structure and Function of Ferritin. Biochem. Educ. 1986, 14 (4), 154–162. 10.1016/0307-4412(86)90203-7. - DOI
-
- Li J. Y.; Paragas N.; Ned R. M.; Qiu A.; Viltard M.; Leete T.; Drexler I. R.; Chen X.; Sanna-Cherchi S.; Mohammed F.; Williams D.; Lin C. S.; Schmidt-Ott K. M.; Andrews N. C.; Barasch J. Scara5 Is a Ferritin Receptor Mediating Non-Transferrin Iron Delivery. Dev. Cell 2009, 16 (1), 35–46. 10.1016/j.devcel.2008.12.002. - DOI - PMC - PubMed
-
- Li L.; Fang C. J.; Ryan J. C.; Niemi E. C.; Lebrón J. A.; Björkman P. J.; Arase H.; Torti F. M.; Torti S. V.; Nakamura M. C.; Seaman W. E. Binding and Uptake of H-Ferritin Are Mediated by Human Transferrin Receptor-1. Proc. Natl. Acad. Sci. U.S.A. 2010, 107 (8), 3505–3510. 10.1073/pnas.0913192107. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

