Immunoglobulin replacement vs prophylactic antibiotics for hypogammaglobulinemia secondary to hematological malignancy
- PMID: 38592710
- PMCID: PMC11006812
- DOI: 10.1182/bloodadvances.2023011231
Immunoglobulin replacement vs prophylactic antibiotics for hypogammaglobulinemia secondary to hematological malignancy
Abstract
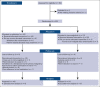
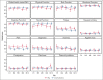
Immunoglobulin replacement and prophylactic antibiotics are commonly used to prevent infections in patients with secondary hypogammaglobulinemia due to hematological malignancies but have never been directly compared. In this randomized controlled feasibility trial conducted in 7 hospitals in Australia and New Zealand, we enrolled patients with secondary hypogammaglobulinemia with either a history of recurrent/severe infection or an immunoglobulin G level <4 g/L. Participants were randomized in a 1:2 ratio to immunoglobulin (0.4 g/kg per 4 weeks IV) or daily antibiotics (trimethoprim-sulfamethoxazole 160 mg/800 mg or, if contraindicated, 100 mg doxycycline) for 12 months. Participants allocated to antibiotics were allowed to crossover after grade ≥3 infections. The primary outcome was proportion of patients alive on the assigned treatment 12 months after randomization. Between August 2017 and April 2019, 63 patients were randomized: 42 to antibiotics and 21 to immunoglobulin. Proportion of participants alive on allocated treatment at 12 months was 76% in the immunoglobulin and 71% in the antibiotic arm (Fisher exact test P=.77; odds ratio, 0.78; 95% CI, 0.22-2.52). The lower quartile for time to first major infection (median, not reached) was 11.1 months for the immunoglobulin and 9.7 months for the antibiotic arm (log-rank test, P=.65). Three participants in the immunoglobulin and 2 in the antibiotic arm had grade ≥3 treatment-related adverse events. A similar proportion of participants remained on antibiotic prophylaxis at 12 months to those on immunoglobulin, with similar rates of major infections. Our findings support the feasibility of progressing to a phase 3 trial. Trial registration #ACTRN12616001723471.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.M.W. is a grant holder. E.M.W. and Z.K.M. received grant funding from CSL Behring, not related to this study. The remaining authors declare no competing financial interests.
A complete list of the participating sites and site investigators from the Australasian Leukaemia and Lymphoma Group appears in the supplemental Material.
Figures

References
-
- Crassini KR, Zhang E, Balendran S, et al. Humoral immune failure defined by immunoglobulin class and immunoglobulin G subclass deficiency is associated with shorter treatment-free and overall survival in chronic lymphocytic leukaemia. Br J Haematol. 2018;181(1):97–101. - PubMed
-
- Dhalla F, Lucas M, Schuh A, et al. Antibody deficiency secondary to chronic lymphocytic leukemia: should patients be treated with prophylactic replacement immunoglobulin? J Clin Immunol. 2014;34(3):277–282. - PubMed
-
- UK DoH NHS England updated commissioning criteria for the use of therapeutic immunoglobulin (Ig) in immunology, haematology, neurology and infectious diseases in England. 2019. http://igd.mdsas.com/wp-content/uploads/Ig-PWG-Guidance-for-the-use-of-I...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

