Anti-EGFR Rechallenge in Patients With Refractory ctDNA RAS/BRAF wt Metastatic Colorectal Cancer: A Nonrandomized Controlled Trial
- PMID: 38592721
- PMCID: PMC11004834
- DOI: 10.1001/jamanetworkopen.2024.5635
Anti-EGFR Rechallenge in Patients With Refractory ctDNA RAS/BRAF wt Metastatic Colorectal Cancer: A Nonrandomized Controlled Trial
Erratum in
-
Error in Author Affiliation.JAMA Netw Open. 2024 May 1;7(5):e2418437. doi: 10.1001/jamanetworkopen.2024.18437. JAMA Netw Open. 2024. PMID: 38776089 Free PMC article. No abstract available.
Abstract
Importance: The available evidence regarding anti-epidermal growth factor receptor (EGFR) inhibitor rechallenge in patients with refractory circulating tumor DNA (ctDNA) RAS/BRAF wild-type (wt) metastatic colorectal cancer (mCRC) is derived from small retrospective and prospective studies.
Objective: To evaluate the efficacy of anti-EGFR rechallenge in patients with refractory ctDNA RAS/BRAF wt mCRC.
Design, setting, and participants: This nonrandomized controlled trial used a pooled analysis of individual patient data from patients with RAS/BRAF wt ctDNA mCRC enrolled in 4 Italian trials (CAVE, VELO, CRICKET, and CHRONOS) and treated with anti-EGFR rechallenge between 2015 and 2022 (median [IQR] follow-up, 28.1 [25.8-35.0] months).
Intervention: Patients received anti-EGFR rechallenge therapy, including cetuximab plus avelumab, trifluridine-tipiracil plus panitumumab, irinotecan plus cetuximab, or panitumumab monotherapy.
Main outcomes and measures: Overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and disease control rate (DCR) were calculated. Exploratory subgroup analysis evaluating several clinical variables was performed. Safety was reported.
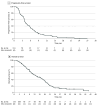
Results: Overall, 114 patients with RAS/BRAF wt ctDNA mCRC (median [IQR] age, 61 [29-88] years; 66 men [57.9%]) who received anti-EGFR rechallenge as experimental therapy (48 received cetuximab plus avelumab, 26 received trifluridine-tipiracil plus panitumumab, 13 received irinotecan plus cetuximab, and 27 received panitumumab monotherapy) were included in the current analysis. Eighty-three patients (72.8%) had received 2 previous lines of therapy, and 31 patients (27.2%) had received 3 or more previous lines of therapy. The ORR was 17.5% (20 patients), and the DCR was 72.3% (82 patients). The median PFS was 4.0 months (95% CI, 3.2-4.7 months), and the median OS was 13.1 months (95% CI, 9.5-16.7 months). The subgroup of patients without liver involvement had better clinical outcomes. The median PFS was 5.7 months (95% CI, 4.8-6.7 months) in patients without liver metastasis compared with 3.6 months (95% CI, 3.3-3.9 months) in patients with liver metastasis (hazard ratio, 0.56; 95% CI, 0.37-0.83; P = .004). The median OS was 17.7 months (95% CI, 13-22.4 months) in patients without liver metastasis compared with 11.5 months (95% CI, 9.3-13.9 months) in patients with liver metastasis (hazard ratio, 0.63; 95% CI, 0.41-0.97; P = .04). Treatments showed manageable toxic effects.
Conclusions and relevance: These findings suggest that anti-EGFR rechallenge therapy has promising antitumor activity in patients with refractory ctDNA RAS/BRAF wt mCRC. Within the limitation of a subgroup analysis, the absence of liver metastases was associated with significant improved survival.
Trial registration: ClinicalTrials.gov Identifiers: NCT02296203; NCT04561336; NCT03227926; NCT05468892.
Conflict of interest statement
Figures

References
-
- Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4(11):1269-1280. doi:10.1158/2159-8290.CD-14-0462 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

