Morphological, Anatomical, and Physiological Characteristics of Heteroblastic Acacia melanoxylon Grown under Weak Light
- PMID: 38592868
- PMCID: PMC10974800
- DOI: 10.3390/plants13060870
Morphological, Anatomical, and Physiological Characteristics of Heteroblastic Acacia melanoxylon Grown under Weak Light
Abstract
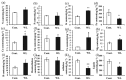
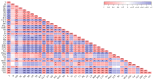
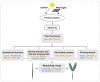
Acacia melanoxylon is a fast-growing macrophanerophyte with strong adaptability whose leaf enables heteromorphic development. Light is one of the essential environmental factors that induces the development of the heteroblastic leaf of A. melanoxylon, but its mechanism is unclear. In this study, the seedlings of A. melanoxylon clones were treated with weak light (shading net with 40% of regular light transmittance) and normal light (control) conditions for 90 d and a follow-up observation. The results show that the seedlings' growth and biomass accumulation were inhibited under weak light. After 60 days of treatment, phyllodes were raised under the control condition while the remaining compound was raised under weak light. The balance of root, stem, and leaf biomass changed to 15:11:74 under weak light, while it was 40:15:45 under control conditions. After comparing the anatomical structures of the compound leaves and phyllode, they were shown to have their own strategies for staying hydrated, while phyllodes were more able to control water loss and adapt to intense light. The compound leaves exhibited elevated levels of K, Cu, Ca, and Mg, increased antioxidant enzyme activity and proline content, and higher concentrations of chlorophyll a, carotenoids, ABA, CTK, and GA. However, they displayed a relatively limited photosynthetic capacity. Phyllodes exhibited higher levels of Fe, cellulose, lignin, IAA content, and high photosynthetic capacity with a higher maximum net photosynthetic rate, light compensation point, dark respiration rate, and water use efficiency. The comparative analysis of compound leaves and phyllodes provides a basis for understanding the diverse survival strategies that heteroblastic plants employ to adapt to environmental changes.
Keywords: Acacia melanoxylon; heteroblasty; phenotypic plasticity; photosynthesis; phyllodes; stress.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


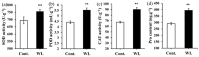


Similar articles
-
Optimal allocation of resources in response to shading and neighbours in the heteroblastic species, Acacia implexa.Ann Bot. 2011 Feb;107(2):219-28. doi: 10.1093/aob/mcq228. Epub 2010 Dec 6. Ann Bot. 2011. PMID: 21135029 Free PMC article.
-
Heteroblastic development and the optimal partitioning of traits among contrasting environments in Acacia implexa.Ann Bot. 2009 Jan;103(1):95-105. doi: 10.1093/aob/mcn210. Epub 2008 Nov 1. Ann Bot. 2009. PMID: 18978364 Free PMC article.
-
Functional trait divergence associated with heteromorphic leaves in a climbing fig.Front Plant Sci. 2023 Sep 19;14:1261240. doi: 10.3389/fpls.2023.1261240. eCollection 2023. Front Plant Sci. 2023. PMID: 37794929 Free PMC article.
-
[Effects of shading on chlorophyll content and photosynthetic characteristics in leaves of Phoebe bournei.].Ying Yong Sheng Tai Xue Bao. 2019 Sep;30(9):2941-2948. doi: 10.13287/j.1001-9332.201909.002. Ying Yong Sheng Tai Xue Bao. 2019. PMID: 31529868 Chinese.
-
Physiological and molecular mechanisms of Acacia melanoxylon stem in response to boron deficiency.Front Plant Sci. 2023 Oct 27;14:1268835. doi: 10.3389/fpls.2023.1268835. eCollection 2023. Front Plant Sci. 2023. PMID: 37964998 Free PMC article.
Cited by
-
Physiological and transcriptomic analyses reveal the regulatory mechanisms for the adaptation of Quercus robur to shade conditions.BMC Plant Biol. 2025 Jul 2;25(1):821. doi: 10.1186/s12870-025-06843-w. BMC Plant Biol. 2025. PMID: 40604453 Free PMC article.
-
Phyllotaxy and environmental factors influences on leaf trait dimensions in Fraxinus mandshurica: a multidimensional approach within temperate forests.Front Plant Sci. 2025 Jul 15;16:1626579. doi: 10.3389/fpls.2025.1626579. eCollection 2025. Front Plant Sci. 2025. PMID: 40734989 Free PMC article.
References
-
- Tanaka-Oda A., Kenzo T., Kashimura S., Ninomiya I., Wang L., Yoshikawa K., Fukuda K. Physiological and morphological differences in the heterophylly of Sabina vulgaris Ant. in the semi-arid environment of Mu Us Desert, Inner Mongolia, China. J. Arid Environ. 2010;74:43–48. doi: 10.1016/j.jaridenv.2009.07.013. - DOI
-
- Hamilton M., Tilyard P., Williams D., Vaillancourt R., Wardlaw T., Potts B. The genetic variation in the timing of heteroblastic transition in Eucalyptus globulus is stable across environments. Aust. J. Bot. 2011;59:170–175. doi: 10.1071/BT10313. - DOI
-
- Deschamp P.A., Cooke T.J. Causal mechanisms of leaf dimorphism in the aquatic angiosperm Callitriche heterophylla. Am. J. Bot. 1984;71:319–329. doi: 10.1002/j.1537-2197.1984.tb12520.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

