Removal of phosphoglycolate in hyperthermophilic archaea
- PMID: 38593075
- PMCID: PMC11032457
- DOI: 10.1073/pnas.2311390121
Removal of phosphoglycolate in hyperthermophilic archaea
Abstract
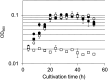
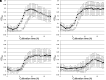
Many organisms that utilize the Calvin-Benson-Bassham (CBB) cycle for autotrophic growth harbor metabolic pathways to remove and/or salvage 2-phosphoglycolate, the product of the oxygenase activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). It has been presumed that the occurrence of 2-phosphoglycolate salvage is linked to the CBB cycle, and in particular, the C2 pathway to the CBB cycle and oxygenic photosynthesis. Here, we examined 2-phosphoglycolate salvage in the hyperthermophilic archaeon Thermococcus kodakarensis, an obligate anaerobe that harbors a Rubisco that functions in the pentose bisphosphate pathway. T. kodakarensis harbors enzymes that have the potential to convert 2-phosphoglycolate to glycine and serine, and their genes were identified by biochemical and/or genetic analyses. 2-phosphoglycolate phosphatase activity increased 1.6-fold when cells were grown under microaerobic conditions compared to anaerobic conditions. Among two candidates, TK1734 encoded a phosphatase specific for 2-phosphoglycolate, and the enzyme was responsible for 80% of the 2-phosphoglycolate phosphatase activity in T. kodakarensis cells. The TK1734 disruption strain displayed growth impairment under microaerobic conditions, which was relieved upon addition of sodium sulfide. In addition, glycolate was detected in the medium when T. kodakarensis was grown under microaerobic conditions. The results suggest that T. kodakarensis removes 2-phosphoglycolate via a phosphatase reaction followed by secretion of glycolate to the medium. As the Rubisco in T. kodakarensis functions in the pentose bisphosphate pathway and not in the CBB cycle, mechanisms to remove 2-phosphoglycolate in this archaeon emerged independent of the CBB cycle.
Keywords: 2-phosphoglycolate; Archaea; Rubisco; metabolism.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO)-mediated de novo synthesis of glycolate-based polyhydroxyalkanoate in Escherichia coli.J Biosci Bioeng. 2019 Sep;128(3):302-306. doi: 10.1016/j.jbiosc.2019.03.002. Epub 2019 Apr 12. J Biosci Bioeng. 2019. PMID: 30987875
-
A native phosphoglycolate salvage pathway of the synthetic autotrophic yeast Komagataella phaffii.Microlife. 2023 Dec 11;5:uqad046. doi: 10.1093/femsml/uqad046. eCollection 2024. Microlife. 2023. PMID: 38234447 Free PMC article.
-
Phosphoglycolate salvage in a chemolithoautotroph using the Calvin cycle.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22452-22461. doi: 10.1073/pnas.2012288117. Epub 2020 Aug 20. Proc Natl Acad Sci U S A. 2020. PMID: 32820073 Free PMC article.
-
Photorespiratory glycolate-glyoxylate metabolism.J Exp Bot. 2016 May;67(10):3041-52. doi: 10.1093/jxb/erw090. Epub 2016 Mar 19. J Exp Bot. 2016. PMID: 26994478 Review.
-
Rubisco Function, Evolution, and Engineering.Annu Rev Biochem. 2023 Jun 20;92:385-410. doi: 10.1146/annurev-biochem-040320-101244. Epub 2023 Apr 26. Annu Rev Biochem. 2023. PMID: 37127263 Review.
Cited by
-
Chromosomal domain formation by archaeal SMC, a roadblock protein, and DNA structure.Nat Commun. 2025 Feb 19;16(1):1312. doi: 10.1038/s41467-025-56197-y. Nat Commun. 2025. PMID: 39971902 Free PMC article.
-
TK2268 encodes the major aminotransferase involved in the conversion from oxaloacetic acid to aspartic acid in Thermococcus kodakarensis.Appl Environ Microbiol. 2025 Mar 19;91(3):e0201724. doi: 10.1128/aem.02017-24. Epub 2025 Feb 24. Appl Environ Microbiol. 2025. PMID: 39992121 Free PMC article.
References
-
- Bassham J. A., Benson A. A., Calvin M., The path of carbon in photosynthesis. J. Biol. Chem. 185, 781–787 (1950). - PubMed
-
- Hartman F. C., Harpel M. R., Structure, function, regulation, and assembly of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Annu. Rev. Biochem. 63, 197–234 (1994). - PubMed
-
- Shively J. M., van Keulen G., Meijer W. G., Something from almost nothing: Carbon dioxide fixation in chemoautotrophs. Annu. Rev. Microbiol. 52, 191–230 (1998). - PubMed
-
- Tolbert N. E., The C2 oxidative photosynthetic carbon cycle. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 48, 1–25 (1997). - PubMed
-
- Anderson L. E., Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim. Biophys. Acta 235, 237–244 (1971). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

