A systematic review and meta-analysis of factors related to first line drugs refractoriness in patients with juvenile myoclonic epilepsy (JME)
- PMID: 38593118
- PMCID: PMC11003615
- DOI: 10.1371/journal.pone.0300930
A systematic review and meta-analysis of factors related to first line drugs refractoriness in patients with juvenile myoclonic epilepsy (JME)
Abstract
Introduction: Juvenile Myoclonic Epilepsy (JME) is a prevalent form of epileptic disorder, specifically categorized within the realm of Genetic Generalized Epilepsy (GGE). Its hallmark features encompass unprovoked bilateral myoclonus and tonic-clonic seizures that manifest during adolescence. While most JME patients respond favorably to anti-seizure medication (ASM), a subset experiences refractory JME, a condition where seizures persist despite rigorous ASM treatment, often termed "Drug-Resistant Epilepsy" (DRE). This systematic review and meta-analysis aims to determine the prevalence of refractory JME, and further to identify socio-demographic, electrophysiological and clinical risk factors associated with its occurrence. Pinpointing these factors is crucial as it offers the potential to predict ASM responsiveness, enabling early interventions and tailored care strategies for patients.
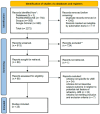
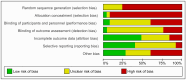
Material and methods: The systematic review and meta-analysis followed the Cochrane Handbook and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study evaluated outcomes post ASM treatment in JME cohorts by searching papers published up to September 2023 in PubMed/MEDLINE, Scopus, and Google Scholar databases. Predefined inclusion criteria were met by 25 eligible studies, forming the basis for analysis.
Results: A total of 22 potential risk factors for refractory JME were documented. Notably, robust risk factors for treatment resistance included Psychiatric Disorder (Odds Ratio (OR), 3.42 [2.54, 4.61] (95% Confidence Inverval (Cl)), Febrile Seizures (OR, 1.83 [1.14, 2.96] (95% Cl)), Alcohol Consumption (OR, 16.86 [1.94, 146.88] (95%Cl)), Aura (OR, 2.15 [1.04, 4.47] (95%Cl)), childhood absence epilepsy (CAE) evolving into JME (OR, 4.54 [1.61, 12.78] (95%CI)), occurrence of three seizure types (OR, 2.96 [1.96, 4.46] (95%CI)), and Focal EEG abnormalities (OR, 1.85 [1.13, 3.01] (95%Cl)). In addition, there were some non-significant risk factors for DRE because of noticeable heterogeneity.
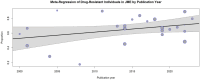
Conclusion: In aggregate, over 36% of JME patients demonstrated drug resistance, with seven significant risk factors closely linked to this refractoriness. The interplay between these factors and whether they denote treatment non-response or heightened disease burden remains an open question and more studies would be required to fully examine their influence.
Copyright: © 2024 Fayad et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Refractory juvenile myoclonic epilepsy: a meta-analysis of prevalence and risk factors.Eur J Neurol. 2019 Jun;26(6):856-864. doi: 10.1111/ene.13811. Epub 2018 Oct 7. Eur J Neurol. 2019. PMID: 30223294 Free PMC article.
-
Juvenile myoclonic epilepsy refractory to treatment in a tertiary referral center.Epilepsy Behav. 2018 May;82:81-86. doi: 10.1016/j.yebeh.2018.03.002. Epub 2018 Mar 27. Epilepsy Behav. 2018. PMID: 29602081
-
Juvenile myoclonic epilepsy subsyndromes: family studies and long-term follow-up.Brain. 2006 May;129(Pt 5):1269-80. doi: 10.1093/brain/awl048. Epub 2006 Mar 6. Brain. 2006. PMID: 16520331
-
Topiramate for juvenile myoclonic epilepsy.Cochrane Database Syst Rev. 2019 Jan 28;1(1):CD010008. doi: 10.1002/14651858.CD010008.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 24;11:CD010008. doi: 10.1002/14651858.CD010008.pub5. PMID: 30687937 Free PMC article. Updated.
-
[Juvenile myoclonic epilepsy].Tidsskr Nor Laegeforen. 2012 Aug 7;132(14):1610-3. doi: 10.4045/tidsskr.11.1518. Tidsskr Nor Laegeforen. 2012. PMID: 22875125 Review. Norwegian.
Cited by
-
Seizure freedom and therapy discontinuation in patients with idiopathic generalized epilepsy: retrospective cohort study from a tertiary epilepsy outpatient service.J Neurol. 2025 Feb 22;272(3):218. doi: 10.1007/s00415-025-12890-y. J Neurol. 2025. PMID: 39985574
-
Development and Validation of Artificial Intelligence Models for Prognosis Prediction of Juvenile Myoclonic Epilepsy with Clinical and Radiological Features.J Clin Med. 2024 Aug 27;13(17):5080. doi: 10.3390/jcm13175080. J Clin Med. 2024. PMID: 39274294 Free PMC article.
References
-
- Fisher RS, Boas WVE, Blume W, Elger C, Genton P, Lee P, et al.. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x - DOI - PubMed
-
- Lee GP. Neuropsychology of epilepsy and epilepsy surgery. Oxford workshop series. Oxford; New York: Oxford University Press; 2010.
-
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al.. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. The Lancet. 2007;369(9566):1016–1026. doi: 10.1016/S0140-6736(07)60460-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

