Elevated interleukin-8 expression by skin fibroblasts as a potential contributor to pain in women with Fabry disease
- PMID: 38593151
- PMCID: PMC11003625
- DOI: 10.1371/journal.pone.0300687
Elevated interleukin-8 expression by skin fibroblasts as a potential contributor to pain in women with Fabry disease
Abstract
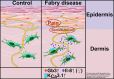
Fabry disease (FD) is a lysosomal storage disorder of X-linked inheritance. Mutations in the α-galactosidase A gene lead to cellular globotriaosylceramide (Gb3) depositions and triggerable acral burning pain in both sexes as an early FD symptom of unknown pathophysiology. We aimed at elucidating the link between skin cells and nociceptor sensitization contributing to FD pain in a sex-associated manner. We used cultured keratinocytes and fibroblasts of 27 adult FD patients and 20 healthy controls. Epidermal keratinocytes and dermal fibroblasts were cultured and immunoreacted to evaluate Gb3 load. Gene expression analysis of pain-related ion channels and pro-inflammatory cytokines was performed in dermal fibroblasts. We further investigated electrophysiological properties of induced pluripotent stem cell (iPSC) derived sensory-like neurons of a man with FD and a healthy man and incubated the cells with interleukin 8 (IL-8) or fibroblast supernatant as an in vitro model system. Keratinocytes displayed no intracellular, but membrane-bound Gb3 deposits. In contrast, fibroblasts showed intracellular Gb3 and revealed higher gene expression of potassium intermediate/small conductance calcium-activated potassium channel 3.1 (KCa 3.1, KCNN4) in both, men and women with FD compared to controls. Additionally, cytokine expression analysis showed increased IL-8 RNA levels only in female FD fibroblasts. Patch-clamp studies revealed reduced rheobase currents for both iPSC neuron cell lines incubated with IL-8 or fibroblast supernatant of women with FD. We conclude that Gb3 deposition in female FD patient skin fibroblasts may lead to increased KCa3.1 activity and IL-8 secretion. This may result in cutaneous nociceptor sensitization as a potential mechanism contributing to a sex-associated FD pain phenotype.
Copyright: © 2024 Hofmann et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

