Serum chemistry profiling and prognostication in systemic mastocytosis: a registry-based study of the ECNM and GREM
- PMID: 38593217
- PMCID: PMC11214361
- DOI: 10.1182/bloodadvances.2024012756
Serum chemistry profiling and prognostication in systemic mastocytosis: a registry-based study of the ECNM and GREM
Abstract
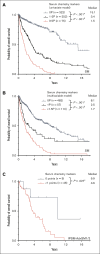
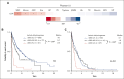
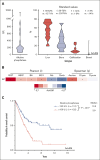
Certain laboratory abnormalities correlate with subvariants of systemic mastocytosis (SM) and are often prognostically relevant. To assess the diagnostic and prognostic value of individual serum chemistry parameters in SM, 2607 patients enrolled within the European Competence Network on Mastocytosis and 575 patients enrolled within the German Registry on Eosinophils and Mast Cells were analyzed. For screening and diagnosis of SM, tryptase was identified as the most specific serum parameter. For differentiation between indolent and advanced SM (AdvSM), the following serum parameters were most relevant: tryptase, alkaline phosphatase, β2-microglobulin, lactate dehydrogenase (LDH), albumin, vitamin B12, and C-reactive protein (P < .001). With regard to subvariants of AdvSM, an elevated LDH of ≥260 U/L was associated with multilineage expansion (leukocytosis, r = 0.37, P < .001; monocytosis, r = 0.26, P < .001) and the presence of an associated myeloid neoplasm (P < .001), whereas tryptase levels were highest in mast cell leukemia (MCL) vs non-MCL (308μg/L vs 146μg/L, P = .003). Based on multivariable analysis, the hazard-risk weighted assignment of 1 point to LDH (hazard ratio [HR], 2.1; 95% confidence interval [CI], 1.1-4.0; P = .018) and 1.5 points each to β2-microglobulin (HR, 2.7; 95% CI, 1.4-5.4; P = .004) and albumin (HR, 3.3; 95% CI, 1.7-6.5; P = .001) delineated a highly predictive 3-tier risk classification system (0 points, 8.1 years vs 1 point, 2.5 years; ≥1.5 points, 1.7 years; P < .001). Moreover, serum chemistry parameters enabled further stratification of patients classified as having an International Prognostic Scoring System for Mastocytosis-AdvSM1/2 risk score (P = .027). In conclusion, serum chemistry profiling is a crucial tool in the clinical practice supporting diagnosis and prognostication of SM and its subvariants.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no conflict of interest within this study and declare the following conflict of interests outside of this study. D.C. received consulting fees from Blueprint Medicines, Novartis, Pfizer and BeiGene, honoraria from Blueprint Medicines, Novartis and AstraZeneca and travel support from Amgen and Janssen. H.N.G.O.E. serves on the advisory board of, and received honoraria from, Blueprint Medicines. R.Z. serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, and Cogent Biosciences. P.B. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis. K.S. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis; and is a member of the study steering committee for the CGT9486 Study of Cogent Biosciences. C.E. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Gilead. K.B. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis. V.S. serves on the advisory board of Blueprint Medicines and Novartis. K.H. received research funding from ThermoFisher; and serves on the advisory board of, and received honoraria from ALK-Abello, Allergopharma, Blueprint, Deciphera, Leo Pharma, Menarini, Novartis, Pfizer, Sanofi, Takeda, and ThermoFisher. M.T. serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, and Cogent. J.G. received research grants (funds for administration of clinical trials) from Novartis, Blueprint Medicines, and Cogent Biosciences; serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, Deciphera, and Cogent Biosciences; and received reimbursement of travel expenses from Novartis and Blueprint Medicines. M.A. received research grants from Blueprint Medicines; and serves on the advisory board of, and received honoraria from, AB Science, Blueprint Medicines, Novartis, and ThermoFisher. H.C.K.-N. served as a nonpaid independent monitoring committee member of the avapritinib study. J.P. serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, and Deciphera; is a member of the study steering committee for the HARBOR Study of BLU-263 for indolent systemic mastocytosis. W.R.S. serves on the advisory board of, and received honoraria from, AbbVie, Novartis, Bristol Myers Squibb/Celgene, Pfizer, Teva, and Stemline. P.V. serves on the advisory board of, and received honoraria from, Novartis, Blueprint, Bristol Myers Squibb/Celgene, Pfizer, Incyte, AOP Orphan, Cogent, and Stemline. A.R. is a member of the study steering committee for the global trial of midostaurin in AdvSM (Novartis); is a member of the response adjudication committee for studies of avapritinib in AdvSM (Blueprint Medicines), and the study steering committee for the phase 2 trial of ripretinib in AdvSM (Deciphera Pharmaceuticals); has received funding for the conduct of these trials; and has received honoraria and reimbursement of travel expenses from Novartis, Blueprint Medicines, and Deciphera Pharmaceuticals. J.S. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis. The remaining authors declare no competing financial interests.
Figures


References
-
- Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–625. - PubMed
-
- Reiter A, George TI, Gotlib J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood. 2020;135(16):1365–1376. - PubMed
-
- Erben P, Schwaab J, Metzgeroth G, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93(1):81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

