Proton FLASH Radiotherapy Ameliorates Radiation-induced Salivary Gland Dysfunction and Oral Mucositis and Increases Survival in a Mouse Model of Head and Neck Cancer
- PMID: 38593239
- PMCID: PMC11148539
- DOI: 10.1158/1535-7163.MCT-23-0663
Proton FLASH Radiotherapy Ameliorates Radiation-induced Salivary Gland Dysfunction and Oral Mucositis and Increases Survival in a Mouse Model of Head and Neck Cancer
Abstract
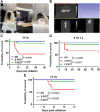
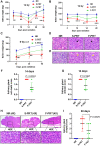
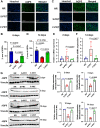
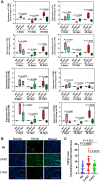
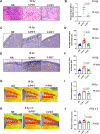
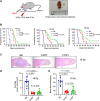
Head and neck cancer radiotherapy often damages salivary glands and oral mucosa, severely negatively impacting patients' quality of life. The ability of FLASH proton radiotherapy (F-PRT) to decrease normal tissue toxicity while maintaining tumor control compared with standard proton radiotherapy (S-PRT) has been previously demonstrated for several tissues. However, its potential in ameliorating radiation-induced salivary gland dysfunction and oral mucositis and controlling orthotopic head and neck tumor growth has not been reported. The head and neck area of C57BL/6 mice was irradiated with a single dose of radiotherapy (ranging from 14-18 Gy) or a fractionated dose of 8 Gy × 3 of F-PRT (128 Gy/second) or S-PRT (0.95 Gy/second). Following irradiation, the mice were studied for radiation-induced xerostomia by measuring their salivary flow. Oral mucositis was analyzed by histopathologic examination. To determine the ability of F-PRT to control orthotopic head and neck tumors, tongue tumors were generated in the mice and then irradiated with either F-PRT or S-PRT. Mice treated with either a single dose or fractionated dose of F-PRT showed significantly improved survival than those irradiated with S-PRT. F-PRT-treated mice showed improvement in their salivary flow. S-PRT-irradiated mice demonstrated increased fibrosis in their tongue epithelium. F-PRT significantly increased the overall survival of the mice with orthotopic tumors compared with the S-PRT-treated mice. The demonstration that F-PRT decreases radiation-induced normal tissue toxicity without compromising tumor control, suggests that this modality could be useful for the clinical management of patients with head and neck cancer.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

