RASP: Optimal Single Puncta Detection in Complex Cellular Backgrounds
- PMID: 38593280
- PMCID: PMC11033865
- DOI: 10.1021/acs.jpcb.4c00174
RASP: Optimal Single Puncta Detection in Complex Cellular Backgrounds
Abstract
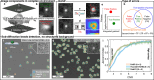
Super-resolution and single-molecule microscopies have been increasingly applied to complex biological systems. A major challenge of these approaches is that fluorescent puncta must be detected in the low signal, high noise, heterogeneous background environments of cells and tissue. We present RASP, Radiality Analysis of Single Puncta, a bioimaging-segmentation method that solves this problem. RASP removes false-positive puncta that other analysis methods detect and detects features over a broad range of spatial scales: from single proteins to complex cell phenotypes. RASP outperforms the state-of-the-art methods in precision and speed using image gradients to separate Gaussian-shaped objects from the background. We demonstrate RASP's power by showing that it can extract spatial correlations between microglia, neurons, and α-synuclein oligomers in the human brain. This sensitive, computationally efficient approach enables fluorescent puncta and cellular features to be distinguished in cellular and tissue environments, with sensitivity down to the level of the single protein. Python and MATLAB codes, enabling users to perform this RASP analysis on their own data, are provided as Supporting Information and links to third-party repositories.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Safieddine A.; Coleno E.; Lionneton F.; Traboulsi A.-M.; Salloum S.; Lecellier C.-H.; Gostan T.; Georget V.; Hassen-Khodja C.; Imbert A.; et al. HT-smFISH: A Cost-Effective and Flexible Workflow for High-Throughput Single-Molecule RNA Imaging. Nat. Protoc. 2023, 18, 157–187. 10.1038/s41596-022-00750-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

