Engineering Nitric Oxide-Releasing Antimicrobial Dental Coating for Targeted Gingival Therapy
- PMID: 38593411
- PMCID: PMC11110066
- DOI: 10.1021/acsabm.4c00051
Engineering Nitric Oxide-Releasing Antimicrobial Dental Coating for Targeted Gingival Therapy
Abstract
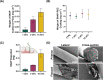
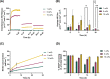
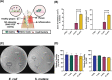
Bacterial biofilms play a central role in the development and progression of periodontitis, a chronic inflammatory condition that affects the oral cavity. One solution to current treatment constraints is using nitric oxide (NO)─with inherent antimicrobial properties. In this study, an antimicrobial coating is developed from the NO donor S-nitroso-N-acetylpenicillamine (SNAP) embedded within polyethylene glycol (PEG) to prevent periodontitis. The SNAP-PEG coating design enabled a controlled NO release, achieving tunable NO levels for more than 24 h. Testing the SNAP-PEG composite on dental floss showed its effectiveness as a uniform and bioactive coating. The coating exhibited antibacterial properties against Streptococcus mutans and Escherichia coli, with inhibition zones measuring up to 7.50 ± 0.28 and 14.80 ± 0.46 mm2, respectively. Furthermore, SNAP-PEG coating materials were found to be stable when stored at room temperature, with 93.65% of SNAP remaining after 28 d. The coatings were biocompatible against HGF and hFOB 1.19 cells through a 24 h controlled release study. This study presents a facile method to utilize controlled NO release with dental antimicrobial coatings comprising SNAP-PEG. This coating can be easily applied to various substrates, providing a user-friendly approach for targeted self-care in managing gingival infections associated with periodontitis.
Keywords: antimicrobial coating; biofilm; dental pathogens; nitric oxide; periodontitis.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Elizabeth Brisbois and Hitesh Handa are co-founders and maintain a financial interest in Nytricx, Inc. The company is exploring possibilities of using nitric oxide releasing materials for medical applications.
Figures

Similar articles
-
Synthesis and Characterization of a Sustained Nitric Oxide-Releasing Orthodontic Elastomeric Chain for Antimicrobial Action.Int J Mol Sci. 2024 Jun 26;25(13):6982. doi: 10.3390/ijms25136982. Int J Mol Sci. 2024. PMID: 39000090 Free PMC article.
-
Long-Term Storage Stability and Nitric Oxide Release Behavior of (N-Acetyl-S-nitrosopenicillaminyl)-S-nitrosopenicillamine-Incorporated Silicone Rubber Coatings.ACS Appl Mater Interfaces. 2022 Jul 13;14(27):30595-30606. doi: 10.1021/acsami.2c06712. Epub 2022 Jun 27. ACS Appl Mater Interfaces. 2022. PMID: 35759508 Free PMC article.
-
Anti-Infective Bacteriophage Immobilized Nitric Oxide-Releasing Surface for Prevention of Thrombosis and Device-Associated Infections.ACS Appl Bio Mater. 2025 Feb 17;8(2):1362-1376. doi: 10.1021/acsabm.4c01638. Epub 2025 Feb 3. ACS Appl Bio Mater. 2025. PMID: 39895136 Free PMC article.
-
Investigation of the susceptibility of clinical infection loads to nitric oxide antibacterial treatment.Nitric Oxide. 2025 Feb;154:19-28. doi: 10.1016/j.niox.2024.11.003. Epub 2024 Nov 17. Nitric Oxide. 2025. PMID: 39561942
-
SNAP@CQD as a promising therapeutic vehicle against HCoVs: An overview.Drug Discov Today. 2023 Jul;28(7):103601. doi: 10.1016/j.drudis.2023.103601. Epub 2023 Apr 28. Drug Discov Today. 2023. PMID: 37119964 Free PMC article. Review.
Cited by
-
Enhancing hand hygiene compliance through the long-lasting antimicrobial effects of nitric oxide-releasing hand sanitizer gel.Biomater Sci. 2025 Jul 8;13(14):3915-3928. doi: 10.1039/d5bm00359h. Biomater Sci. 2025. PMID: 40491350 Free PMC article.
-
Wearable nitric oxide-releasing antibacterial insert for preventing device-associated infections.J Control Release. 2024 Nov;375:667-680. doi: 10.1016/j.jconrel.2024.09.027. Epub 2024 Sep 24. J Control Release. 2024. PMID: 39288891
References
-
- World Health Organization . Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030, 2022.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
