Targeting sinonasal undifferentiated carcinoma with a combinatory immunotherapy approach
- PMID: 38593586
- PMCID: PMC11024348
- DOI: 10.1016/j.tranon.2024.101943
Targeting sinonasal undifferentiated carcinoma with a combinatory immunotherapy approach
Abstract
Purpose: Sinonasal undifferentiated carcinoma (SNUC) is a rare, aggressive malignancy of the sinonasal cavity with poor prognosis and limited treatment options. To investigate the potential for SNUC sensitivity to combinatory immunotherapy, we performed in vitro studies with SNUC cell lines and used multi-spectral immunofluorescence to characterize the in vivo patient SNUC tumor immune microenvironment (TIME).
Experimental design: Human-derived SNUC cell lines were used for in vitro studies of tumor cell susceptibility to natural killer (NK) cell-based immunotherapeutic strategies. Tumor samples from 14 treatment naïve SNUC patients were examined via multi-spectral immunofluorescence and clinical correlations assessed.
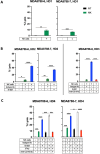
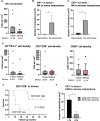
Results: Anti-PD-L1 blockade enhanced NK cell lysis of SNUC cell lines ∼5.4 fold (P ≤ 0.0001). This effect was blocked by a CD16 neutralizing antibody demonstrating activity through an antibody-dependent cellular cytotoxicity (ADCC) mediated pathway. ADCC-dependent lysis of SNUC cells was further enhanced by upregulation of PD-L1 on tumor cells by exogenous interferon-gamma (IFN-γ) administration or interleukin-15 (IL-15) stimulated IFN-γ release from NK cells. Combination treatment with anti-PD-L1 blockade and IL-15 superagonism enhanced NK-cell killing of SNUC cells 9.6-fold (P ≤ 0.0001). Untreated SNUC patient tumor samples were found to have an NK cell infiltrate and PD-L1+ tumor cells at a median of 5.4 cells per mm2. A striking 55.7-fold increase in CKlow tumor cell/NK cell interactions was observed in patients without disease recurrence after treatment (P = 0.022). Patients with higher CD3+CD8+ in the stroma had a significantly improved 5-year overall survival (P = 0.0029) and a significant increase in CKlow tumor cell/CD8+ cytotoxic T cell interactions was noted in long-term survivors (P = 0.0225).
Conclusion: These data provide the pre-clinical rationale for ongoing investigation into combinatory immunotherapy approaches for SNUC.
Keywords: Antibody-dependent cellular cytotoxicity; IL-15; Immune microenvironment; Immunotherapy; Natural killer cells; Sinonasal undifferentiated carcinoma.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest N. London receives research funding from Merck Sharp & Dohme, LLC, regarding HPV-related sinonasal carcinomas not relevant to the present manuscript. P. Soon-Shiong is a founder of ImmunityBio. All other authors declare no competing interests.
Figures



Similar articles
-
Anti-tumor effects of NK cells and anti-PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines.J Immunother Cancer. 2020 Aug;8(2):e000873. doi: 10.1136/jitc-2020-000873. J Immunother Cancer. 2020. PMID: 32830112 Free PMC article.
-
PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations.J Immunother Cancer. 2020 May;8(1):e000450. doi: 10.1136/jitc-2019-000450. J Immunother Cancer. 2020. PMID: 32439799 Free PMC article.
-
Epigenetic and Immune Profile Characteristics in Sinonasal Undifferentiated Carcinoma.Cancer Med. 2024 Nov;13(22):e70413. doi: 10.1002/cam4.70413. Cancer Med. 2024. PMID: 39565059 Free PMC article.
-
Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis.Adv Anat Pathol. 2005 May;12(3):134-43. doi: 10.1097/01.pap.0000163958.29032.56. Adv Anat Pathol. 2005. PMID: 15900114 Review.
-
Potentiation of natural killer cells to overcome cancer resistance to NK cell-based therapy and to enhance antibody-based immunotherapy.Front Immunol. 2023 Nov 24;14:1275904. doi: 10.3389/fimmu.2023.1275904. eCollection 2023. Front Immunol. 2023. PMID: 38077389 Free PMC article. Review.
Cited by
-
Rare Head and Neck Cancers and Pathological Diagnosis Challenges: A Comprehensive Literature Review.Diagnostics (Basel). 2024 Oct 23;14(21):2365. doi: 10.3390/diagnostics14212365. Diagnostics (Basel). 2024. PMID: 39518333 Free PMC article. Review.
-
Complex Craniofacial Cases through Augmented Reality Guidance in Surgical Oncology: A Technical Report.Diagnostics (Basel). 2024 May 27;14(11):1108. doi: 10.3390/diagnostics14111108. Diagnostics (Basel). 2024. PMID: 38893634 Free PMC article.
References
-
- Tyler M.A., Holmes B., Patel Z.M. Oncologic management of sinonasal undifferentiated carcinoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2019;27(1):59–66. - PubMed
-
- London N.R., Jr., Mohyeldin A., Daoud G., Gamez M.E., Blakaj D., Bonomi M., et al. Sinonasal undifferentiated carcinoma: institutional trend toward induction chemotherapy followed by definitive chemoradiation. Head Neck. 2020;42(11):3197–3205. - PubMed
-
- Ejaz A., Wenig B.M. Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv. Anat. Pathol. 2005;12(3):134–143. - PubMed
-
- Abdelmeguid A.S., Bell D., Hanna E.Y. Sinonasal undifferentiated carcinoma. Curr. Oncol. Rep. 2019;21(3):26. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

