Therapeutic nuclear magnetic resonance and intermittent hypoxia trigger time dependent on/off effects in circadian clocks and confirm a central role of superoxide in cellular magnetic field effects
- PMID: 38593630
- PMCID: PMC11016797
- DOI: 10.1016/j.redox.2024.103152
Therapeutic nuclear magnetic resonance and intermittent hypoxia trigger time dependent on/off effects in circadian clocks and confirm a central role of superoxide in cellular magnetic field effects
Abstract
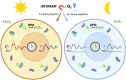
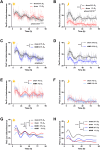
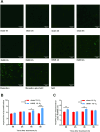
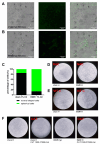
Cellular magnetic field effects are assumed to base on coherent singlet-triplet interconversion of radical pairs that are sensitive to applied radiofrequency (RF) and weak magnetic fields (WEMFs), known as radical pair mechanism (RPM). As a leading model, the RPM explains how quantum effects can influence biochemical and cellular signalling. Consequently, radical pairs generate reactive oxygen species (ROS) that link the RPM to redox processes, such as the response to hypoxia and the circadian clock. Therapeutic nuclear magnetic resonance (tNMR) occupies a unique position in the RPM paradigm because of the used frequencies, which are far below the range of 0.1-100 MHz postulated for the RPM to occur. Nonetheless, tNMR was shown to induce RPM like effects, such as increased extracellular H2O2 levels and altered cellular bioenergetics. In this study we compared the impact of tNMR and intermittent hypoxia on the circadian clock, as well as the role of superoxide in tNMR induced ROS partitioning. We show that both, tNMR and intermittent hypoxia, exert on/off effects on cellular clocks that are dependent on the time of application (day versus night). In addition, our data provide further evidence that superoxide plays a central role in magnetic signal transduction. tNMR used in combination with scavengers, such as Vitamin C, led to strong ROS product redistributions. This discovery might represent the first indication of radical triads in biological systems.
Keywords: Circadian clock; Intermittent hypoxia; Magnetic field effects; Reactive oxygen species; Superoxide.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The study was financed by MedTec Company, Wetzlar Germany, Project Data Base Number 350088.
Figures

Similar articles
-
Little strokes fell big oaks: The use of weak magnetic fields and reactive oxygen species to fight cancer.Redox Biol. 2025 Feb;79:103483. doi: 10.1016/j.redox.2024.103483. Epub 2024 Dec 24. Redox Biol. 2025. PMID: 39729909 Free PMC article. Review.
-
Therapeutic Nuclear Magnetic Resonance affects the core clock mechanism and associated Hypoxia-inducible factor-1.Chronobiol Int. 2021 Aug;38(8):1120-1134. doi: 10.1080/07420528.2021.1910288. Epub 2021 Apr 13. Chronobiol Int. 2021. PMID: 33847185
-
Quantum based effects of therapeutic nuclear magnetic resonance persistently reduce glycolysis.iScience. 2022 Nov 9;25(12):105536. doi: 10.1016/j.isci.2022.105536. eCollection 2022 Dec 22. iScience. 2022. PMID: 36444297 Free PMC article.
-
Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields.PLoS One. 2014 Mar 28;9(3):e93065. doi: 10.1371/journal.pone.0093065. eCollection 2014. PLoS One. 2014. PMID: 24681944 Free PMC article.
-
Redox regulation and pro-oxidant reactions in the physiology of circadian systems.Biochimie. 2016 May;124:178-186. doi: 10.1016/j.biochi.2015.04.014. Epub 2015 Apr 26. Biochimie. 2016. PMID: 25926044 Review.
Cited by
-
Little strokes fell big oaks: The use of weak magnetic fields and reactive oxygen species to fight cancer.Redox Biol. 2025 Feb;79:103483. doi: 10.1016/j.redox.2024.103483. Epub 2024 Dec 24. Redox Biol. 2025. PMID: 39729909 Free PMC article. Review.
-
Magneto-oncology: a radical pair primer.Front Oncol. 2025 Mar 7;15:1539718. doi: 10.3389/fonc.2025.1539718. eCollection 2025. Front Oncol. 2025. PMID: 40123899 Free PMC article. Review.
References
-
- Schulten K., Swenberg C.E., Weiler A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Physikalische Chemie. 1978;111:1–5.
-
- Hore P.J., Mouritsen H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016;45:299–344. - PubMed
-
- Ritz T. Quantum effects in biology: bird navigation. Procedia Chem. 2011;3:262–275.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

