Disease progression, survival, and molecular disparities in Black and White patients with endometrioid endometrial carcinoma in real-world registries and GOG/NRG oncology randomized phase III clinical trials
- PMID: 38593674
- PMCID: PMC11894812
- DOI: 10.1016/j.ygyno.2024.03.026
Disease progression, survival, and molecular disparities in Black and White patients with endometrioid endometrial carcinoma in real-world registries and GOG/NRG oncology randomized phase III clinical trials
Abstract
Objective: Investigate racial disparities in outcomes and molecular features in Black and White patients with endometrioid endometrial carcinoma (EEC).
Methods: Black and White patients diagnosed with EEC who underwent hysterectomy ± adjuvant treatment in SEER, National Cancer Database (NCDB), the Genomics Evidence Neoplasia Information Exchange (GENIE) project (v.13.0), and eight NCI-sponsored randomized phase III clinical trials (RCTs) were studied. Hazard ratio (HR) and 95% confidence interval (CI) were estimated for cancer-related death (CRD), non-cancer death (NCD), and all-cause death.
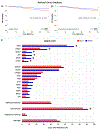
Results: Black (n = 4397) vs. White (n = 47,959) patients in SEER had a HR (95% CI) of 2.04 (1.87-2.23) for CRD and 1.22 (1.09-1.36) for NCD. In NCDB, the HR (95% CI) for death in Black (n = 13,468) vs. White (n = 155,706) patients was 1.52 (1.46-1.58) dropping to 1.29 (1.23-1.36) after propensity-score matching for age, comorbidity, income, insurance, grade, stage, LVSI, and treatment. In GENIE, Black (n = 109) vs. White (n = 1780) patients had fewer PTEN, PIK3R1, FBXW7, NF1, mTOR, CCND1, and PI3K-pathway-related gene mutations. In contrast, TP53 and DNA-repair-related gene mutation frequency as well as tumor mutational burden-high status were similar in Black and White patients. In RCTs, Black (n = 187) vs. White (n = 2877) patients were more likely to have advanced or recurrent disease, higher grade, worse performance status and progressive disease. Risk of death in Black vs. White patients in RCTs was 2.19 (1.77-2.71) persisting to 1.32 (1.09-1.61) after matching for grade, stage, and treatment arm while balancing age and performance status.
Conclusions: Differences exist in clinical presentation, outcomes, and molecular features in Black vs. White patients with EEC in real-world registries and RCTs. Targeted-drug development, strategies to modify social determinants, and diverse inclusion in RCTs are approaches to reduce disparities.
Keywords: AACR GENIE; Endometrioid endometrial cancer; NCDB; Racial disparities; Randomized phase III clinical trial; SEER.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Zachary A. Kopelman, Chunqiao Tian, Jordyn Tumas, Neil T. Phippen, Christopher M. Tarney, Erica R. Hope, Stuart S. Winkler, Suzanne Jokajtys, Calen W. Kucera, Michael T. Richardson, Daniel S. Kapp, Nathaniel L. Jones, Rodney P. Rocconi, John H. Farley, Angeles Alvarez Secord, Casey M. Cosgrove, Matthew A. Powell, Ann Klopp, Joan L Walker, Gini F. Fleming, Nicholas W. Bateman, G. Larry Maxwell, and Kathleen M. Darcy do not have any conflicts to report. John K. Chan reported personal fees from Agenus, AstraZeneca, Eisai, Genmab, GlaxoSmithKline, Immunogen, Mersana, Molecular Targeting Technologies, Myriad, Roche, and Seagen outside the submitted work. Chad A. Hamilton reported personal fees from GlaxoSmithKline outside the submitted work. Charles A. Leath, III received funding from the NIH UG1 CA23330 and P50 CA098252, contracted research with GSK and Merck, and served on a scientific advisory board for GSK and Merck, all outside of the submitted work. Thomas P. Conrads is a ThermoFisher Scientific, Inc. SAB member and receives research funding from AbbVie outside the submitted work.
Figures


Similar articles
-
Disparities in receipt of care for high-grade endometrial cancer: A National Cancer Data Base analysis.Gynecol Oncol. 2017 Apr;145(1):114-121. doi: 10.1016/j.ygyno.2017.01.024. Epub 2017 Feb 1. Gynecol Oncol. 2017. PMID: 28159409
-
Racial disparities in young women with endometrial cancer.Gynecol Oncol. 2018 Mar;148(3):527-534. doi: 10.1016/j.ygyno.2017.12.032. Epub 2018 Jan 5. Gynecol Oncol. 2018. PMID: 29307452 Free PMC article.
-
Impact of hospital volume on racial disparities and outcomes for endometrial cancer.Gynecol Oncol. 2018 May;149(2):329-336. doi: 10.1016/j.ygyno.2018.02.019. Epub 2018 Mar 2. Gynecol Oncol. 2018. PMID: 29506862 Free PMC article.
-
TCGA molecular subgroups of endometrial carcinoma in ovarian endometrioid carcinoma: A quantitative systematic review.Gynecol Oncol. 2021 Nov;163(2):427-432. doi: 10.1016/j.ygyno.2021.08.011. Epub 2021 Aug 24. Gynecol Oncol. 2021. PMID: 34446267
-
Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States.Gynecol Oncol. 2013 Sep;130(3):652-9. doi: 10.1016/j.ygyno.2013.05.020. Epub 2013 May 23. Gynecol Oncol. 2013. PMID: 23707671 Free PMC article. Review.
References
-
- SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute; 2023. Apr 19. [updated: 2023 Nov 16; cited 2024 Jan 2]. Available from: https://seer.cancer.gov/statistics-network/explorer/.
-
- Siegel RL, Giaquinto AN, Jemal A: Cancer statistics, 2024. CA Cancer J Clin 74:12–49, 2024 - PubMed
-
- Bokhman JV: Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10–7, 1983 - PubMed
-
- ACOG.: Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol. 125:1006–26, 2015 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

