Performance Assessment of Treponemal and Nontreponemal Tests for the Diagnosis of Acquired Syphilis
- PMID: 38593786
- PMCID: PMC11154036
- DOI: 10.4269/ajtmh.23-0238
Performance Assessment of Treponemal and Nontreponemal Tests for the Diagnosis of Acquired Syphilis
Abstract
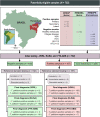
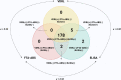
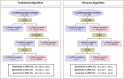
There are a variety of nontreponemal test (NTT) and treponemal test (TT) kits for the serologic diagnosis of syphilis. Because of the complexity of the infection (multiple clinical stages) and the different antigens used in these kits, a systematic evaluation of the accuracy of the currently available commercial tests is warranted. Our objective was to evaluate the performance of commercially available tests for the diagnosis of syphilis infection. In this study, we analyzed one NTT (Venereal Disease Research Laboratory [VDRL] test, Wiener Laboratories, Rosario, Argentina) and two TTs (fluorescent treponemal antibody absorption [FTA-ABS] test, Euroimmun, Lübeck, Germany, and syphilis recombinant ELISA v. 4.0 test [ELISA], Wiener Laboratories, Rosario, Argentina) using a panel of 187 samples, including serum samples from 31 individuals with primary syphilis, 77 with secondary syphilis, and 79 with latent syphilis. An additional 192 samples from uninfected individuals and 323 serum samples from individuals with other diseases were included. The sensitivities of the VDRL, ELISA, and FTA-ABS tests were 97.9%, 100%, and 96.3%, respectively. The VDRL and ELISA tests showed a specificity of 100%, and the FTA-ABS test showed a specificity of 99.5%. Accuracy was 98.9% for the VDRL test, 100% for the ELISA, and 97.9% for the FTA-ABS test. For primary, secondary, and latent syphilis, the ELISA achieved a diagnostic performance of 100%, whereas the sensitivity for the VDRL and FTA-ABS tests ranged from 96.8% to 98.7% and 93.7% to 98.7%, respectively. No difference was observed when the tests were used as traditional or reverse algorithms. In general, all three tests are able to discriminate positive and negative samples for syphilis, regardless of the diagnostic algorithm.
Conflict of interest statement
Disclosure: The present research protocol was approved by the Institutional Research Board for Human Research at the Gonçalo Moniz Institute of the Oswaldo Cruz Foundation (IGM-FIOCRUZ), Salvador, Bahia, Brazil (CAAE number 42341121.0.0000.0040). All procedures were performed in accordance with the principles of the Declaration of Helsinki and its subsequent revisions.
Figures
References
-
- Lawn JE. et al. , 2016. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 387: 587–603. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

