The clinical utility and diagnostic implementation of human subject cell transdifferentiation followed by RNA sequencing
- PMID: 38593811
- PMCID: PMC11080285
- DOI: 10.1016/j.ajhg.2024.03.007
The clinical utility and diagnostic implementation of human subject cell transdifferentiation followed by RNA sequencing
Abstract
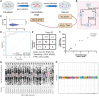
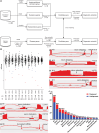
RNA sequencing (RNA-seq) has recently been used in translational research settings to facilitate diagnoses of Mendelian disorders. A significant obstacle for clinical laboratories in adopting RNA-seq is the low or absent expression of a significant number of disease-associated genes/transcripts in clinically accessible samples. As this is especially problematic in neurological diseases, we developed a clinical diagnostic approach that enhanced the detection and evaluation of tissue-specific genes/transcripts through fibroblast-to-neuron cell transdifferentiation. The approach is designed specifically to suit clinical implementation, emphasizing simplicity, cost effectiveness, turnaround time, and reproducibility. For clinical validation, we generated induced neurons (iNeurons) from 71 individuals with primary neurological phenotypes recruited to the Undiagnosed Diseases Network. The overall diagnostic yield was 25.4%. Over a quarter of the diagnostic findings benefited from transdifferentiation and could not be achieved by fibroblast RNA-seq alone. This iNeuron transcriptomic approach can be effectively integrated into diagnostic whole-transcriptome evaluation of individuals with genetic disorders.
Keywords: RNA sequencing; RNA-seq; clinically accessible tissue; fibroblast; genetic diagnosis; induced neuron; isoform; neurological disorder; transcriptome; transdifferentiation.
Copyright © 2024 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Baylor College of Medicine (BCM) and Miraca Holdings Inc. have formed a joint venture with shared ownership and governance of Baylor Genetics (BG), which performs genetic testing and derives revenue. P.L. and C.M.E. are employees of BCM and derive support through a professional services agreement with BG.
Figures



Similar articles
-
RNA variant assessment using transactivation and transdifferentiation.Am J Hum Genet. 2024 Aug 8;111(8):1673-1699. doi: 10.1016/j.ajhg.2024.06.018. Epub 2024 Jul 30. Am J Hum Genet. 2024. PMID: 39084224 Free PMC article.
-
Dissecting direct reprogramming from fibroblast to neuron using single-cell RNA-seq.Nature. 2016 Jun 16;534(7607):391-5. doi: 10.1038/nature18323. Epub 2016 Jun 8. Nature. 2016. PMID: 27281220 Free PMC article.
-
Expanding the Boundaries of RNA Sequencing as a Diagnostic Tool for Rare Mendelian Disease.Am J Hum Genet. 2019 Mar 7;104(3):466-483. doi: 10.1016/j.ajhg.2019.01.012. Epub 2019 Feb 28. Am J Hum Genet. 2019. PMID: 30827497 Free PMC article.
-
Processing and Analysis of RNA-seq Data from Public Resources.Methods Mol Biol. 2021;2243:81-94. doi: 10.1007/978-1-0716-1103-6_4. Methods Mol Biol. 2021. PMID: 33606253 Review.
-
Research Techniques Made Simple: Whole-Transcriptome Sequencing by RNA-Seq for Diagnosis of Monogenic Disorders.J Invest Dermatol. 2020 Jun;140(6):1117-1126.e1. doi: 10.1016/j.jid.2020.02.032. J Invest Dermatol. 2020. PMID: 32446329 Free PMC article. Review.
Cited by
-
Perspective: Pathological transdifferentiation-a novel therapeutic target for cardiovascular diseases and chronic inflammation.Front Cardiovasc Med. 2024 Nov 26;11:1500775. doi: 10.3389/fcvm.2024.1500775. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39660114 Free PMC article.
-
An outlier approach: advancing diagnosis of neurological diseases through integrating proteomics into multi-omics guided exome reanalysis.NPJ Genom Med. 2025 May 3;10(1):36. doi: 10.1038/s41525-025-00493-5. NPJ Genom Med. 2025. PMID: 40319040 Free PMC article.
-
Translating Muscle RNAseq Into the Clinic for the Diagnosis of Muscle Diseases.Ann Clin Transl Neurol. 2025 Jul;12(7):1465-1479. doi: 10.1002/acn3.70078. Epub 2025 May 25. Ann Clin Transl Neurol. 2025. PMID: 40413734 Free PMC article.
-
RNA variant assessment using transactivation and transdifferentiation.Am J Hum Genet. 2024 Aug 8;111(8):1673-1699. doi: 10.1016/j.ajhg.2024.06.018. Epub 2024 Jul 30. Am J Hum Genet. 2024. PMID: 39084224 Free PMC article.
-
Combined genomics and proteomics unveils elusive variants and vast aetiologic heterogeneity in dystonia.Brain. 2025 Aug 1;148(8):2827-2846. doi: 10.1093/brain/awaf059. Brain. 2025. PMID: 39937650 Free PMC article.
References
-
- Splinter K., Adams D.R., Bacino C.A., Bellen H.J., Bernstein J.A., Cheatle-Jarvela A.M., Eng C.M., Esteves C., Gahl W.A., Hamid R., et al. Effect of Genetic Diagnosis on Patients with Previously Undiagnosed Disease. N. Engl. J. Med. 2018;379:2131–2139. doi: 10.1056/NEJMoa1714458. - DOI - PMC - PubMed
-
- Wright C.F., Campbell P., Eberhardt R.Y., Aitken S., Perrett D., Brent S., Danecek P., Gardner E.J., Chundru V.K., Lindsay S.J., et al. Genomic Diagnosis of Rare Pediatric Disease in the United Kingdom and Ireland. N. Engl. J. Med. 2023;388:1559–1571. doi: 10.1056/NEJMoa2209046. - DOI - PMC - PubMed
-
- Lionel A.C., Costain G., Monfared N., Walker S., Reuter M.S., Hosseini S.M., Thiruvahindrapuram B., Merico D., Jobling R., Nalpathamkalam T., et al. Improved diagnostic yield compared with targeted gene sequencing panels suggests a role for whole-genome sequencing as a first-tier genetic test. Genet. Med. 2018;20:435–443. doi: 10.1038/gim.2017.119. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

