Metabolomic profiling of cancer-related fatigue involved in cachexia and chemotherapy
- PMID: 38594321
- PMCID: PMC11004174
- DOI: 10.1038/s41598-024-57747-y
Metabolomic profiling of cancer-related fatigue involved in cachexia and chemotherapy
Abstract
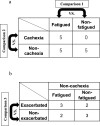
Patients with advanced cancer are frequently burdened with a severe sensation of fatigue called cancer-related fatigue (CRF). CRF is induced at various stages and treatments, such as cachexia and chemotherapy, and reduces the overall survival of patients. Objective and quantitative assessment of CRF could contribute to the diagnosis and prediction of treatment efficacy. However, such studies have not been intensively performed, particularly regarding metabolic profiles. Here, we conducted plasma metabolomics of 15 patients with urological cancer. The patients with and without fatigue, including those with cachexia or chemotherapy-induced fatigue, were compared. Significantly lower concentrations of valine and tryptophan were observed in fatigued patients than in non-fatigued patients. In addition, significantly higher concentrations of polyamine pathway metabolites were observed in patients with fatigue and cachexia than in those without cachexia. Patients with exacerbated fatigue due to chemotherapy showed significantly decreased cysteine and methionine metabolism before chemotherapy compared with those without fatigue exacerbation. These findings suggest that plasma metabolic profiles could help improve the diagnosis and monitoring of CRF.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Cardiovascular training for fatigue in people with cancer.Cochrane Database Syst Rev. 2025 Feb 20;2(2):CD015517. doi: 10.1002/14651858.CD015517. Cochrane Database Syst Rev. 2025. PMID: 39976199
-
Corticosteroids for the management of cancer-related fatigue in adults with advanced cancer.Cochrane Database Syst Rev. 2023 Jan 23;1(1):CD013782. doi: 10.1002/14651858.CD013782.pub2. Cochrane Database Syst Rev. 2023. PMID: 36688471 Free PMC article.
-
Yoga for fatigue in people with cancer.Cochrane Database Syst Rev. 2025 May 27;5(5):CD015520. doi: 10.1002/14651858.CD015520. Cochrane Database Syst Rev. 2025. PMID: 40421669 Review.
Cited by
-
Comparative Efficacy of Various Exercise Types on Cancer-Related Fatigue for Cancer Survivors: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials.Cancer Med. 2025 Apr;14(7):e70816. doi: 10.1002/cam4.70816. Cancer Med. 2025. PMID: 40145635 Free PMC article.
-
Understanding neurocognitive recovery in older adults after total hip arthroplasty-neurocognitive assessment, blood biomarkers and patient experiences: a mixed-methods study.BMJ Open. 2025 Jan 28;15(1):e093872. doi: 10.1136/bmjopen-2024-093872. BMJ Open. 2025. PMID: 39880425 Free PMC article.
-
Effect of different types of aerobic exercises on cancer-related fatigue among colorectal cancer patients: a meta-analysis based on randomized controlled trials.BMC Cancer. 2025 Jul 5;25(1):1145. doi: 10.1186/s12885-025-14532-y. BMC Cancer. 2025. PMID: 40618015 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

