Control of DNA replication in vitro using a reversible replication barrier
- PMID: 38594502
- PMCID: PMC11230854
- DOI: 10.1038/s41596-024-00977-1
Control of DNA replication in vitro using a reversible replication barrier
Abstract
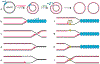
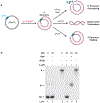
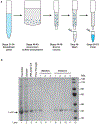
A major obstacle to studying DNA replication is that it involves asynchronous and highly delocalized events. A reversible replication barrier overcomes this limitation and allows replication fork movement to be synchronized and localized, facilitating the study of replication fork function and replication coupled repair. Here we provide details on establishing a reversible replication barrier in vitro and using it to monitor different aspects of DNA replication. DNA template containing an array of lac operator (lacO) sequences is first bound to purified lac repressor (LacR). This substrate is then replicated in vitro using a biochemical replication system, which results in replication forks stalled on either side of the LacR array regardless of when or where they arise. Once replication forks are synchronized at the barrier, isopropyl-β-D-thiogalactopyranoside can be added to disrupt LacR binding so that replication forks synchronously resume synthesis. We describe how this approach can be employed to control replication fork elongation, termination, stalling and uncoupling, as well as assays that can be used to monitor these processes. We also explain how this approach can be adapted to control whether replication forks encounter a DNA lesion on the leading or lagging strand template and whether a converging fork is present. The required reagents can be prepared in 1-2 weeks and experiments using this approach are typically performed over 1-3 d. The main requirements for utilizing the LacR replication barrier are basic biochemical expertise and access to an in vitro system to study DNA replication. Investigators should also be trained in working with radioactive materials.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
No, I declare the authors have no competing interests as defined by Nature Research
Figures






Similar articles
-
Adapting Safety Plans for Autistic Adults with Involvement from the Autism Community.Autism Adulthood. 2025 May 28;7(3):293-302. doi: 10.1089/aut.2023.0124. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539213
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
Cited by
-
Quantifying complexity in DNA structures with high resolution Atomic Force Microscopy.Nat Commun. 2025 Jul 1;16(1):5482. doi: 10.1038/s41467-025-60559-x. Nat Commun. 2025. PMID: 40593613 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32ES007028/U.S. Department of Health & Human Services | NIH | National Institute of Environmental Health Sciences (NIEHS)
- F32 GM148024/GM/NIGMS NIH HHS/United States
- R01ES034847/U.S. Department of Health & Human Services | NIH | National Institute of Environmental Health Sciences (NIEHS)
- R01 ES034847/ES/NIEHS NIH HHS/United States
- T32 ES007028/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources

