Targeting transitioning lung monocytes/macrophages as treatment strategies in lung disease related to environmental exposures
- PMID: 38594676
- PMCID: PMC11003126
- DOI: 10.1186/s12931-024-02804-3
Targeting transitioning lung monocytes/macrophages as treatment strategies in lung disease related to environmental exposures
Abstract
Background: Environmental/occupational exposures cause significant lung diseases. Agricultural organic dust extracts (ODE) and bacterial component lipopolysaccharide (LPS) induce recruited, transitioning murine lung monocytes/macrophages, yet their cellular role remains unclear.
Methods: CCR2 RFP+ mice were intratracheally instilled with high concentration ODE (25%), LPS (10 μg), or gram-positive peptidoglycan (PGN, 100 μg) for monocyte/macrophage cell-trafficking studies. CCR2 knockout (KO) mice and administration of intravenous clodronate liposomes strategies were employed to reduce circulating monocytes available for lung recruitment following LPS exposure. Lung tissues and bronchoalveolar lavage fluid (BALF) were collected. Pro-inflammatory and/or pro-fibrotic cytokines, chemokines, and lung extracellular matrix mediators were quantitated by ELISA. Infiltrating lung cells including monocyte/macrophage subpopulations, neutrophils, and lymphocytes were characterized by flow cytometry. Lung histopathology, collagen content, vimentin, and post-translational protein citrullination and malondialdehyde acetaldehyde (MAA) modification were quantitated. Parametric statistical tests (one-way ANOVA, Tukey'smultiple comparison) and nonparametric statistical (Kruskal-Wallis, Dunn's multiple comparison) tests were used following Shapiro-Wilk testing for normality.
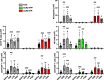
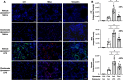
Results: Intratracheal instillation of ODE, LPS, or PGN robustly induced the recruitment of inflammatory CCR2+ CD11cintCD11bhi monocytes/macrophages and both CCR2+ and CCR2- CD11c-CD11bhi monocytes at 48 h. There were also increases in CCR2+ CD4+ and CD8+ T cells and NK cells. Despite reductions in LPS-induced lung infiltrating CD11cintCD11bhi cells (54% reduction), CCR2 knockout (KO) mice were not protected against LPS-induced inflammatory and pro-fibrotic consequences. Instead, compensatory increases in lung neutrophils and CCL2 and CCL7 release occurred. In contrast, the depletion of circulating monocytes through the administration of intravenous clodronate (vs. vehicle) liposomes 24 h prior to LPS exposure reduced LPS-induced infiltrating CD11cintCD11bhi monocyte-macrophage subpopulation by 59% without compensatory changes in other cell populations. Clodronate liposome pre-treatment significantly reduced LPS-induced IL-6 (66% reduction), matrix metalloproteinases (MMP)-3 (36%), MMP-8 (57%), tissue inhibitor of metalloproteinases (61%), fibronectin (38%), collagen content (22%), and vimentin (40%). LPS-induced lung protein citrullination and MAA modification, post-translational modifications implicated in lung disease, were reduced (39% and 48%) with clodronate vs. vehicle liposome.
Conclusion: Highly concentrated environmental/occupational exposures induced the recruitment of CCR2+ and CCR2- transitioning monocyte-macrophage and monocyte subpopulations and targeting peripheral monocytes may reduce the adverse lung consequences resulting from exposures to LPS-enriched inhalants.
Keywords: Endotoxin; Immunology; Inflammation; Lung disease; Monocytes; Organic dust.
© 2024. The Author(s).
Conflict of interest statement
JAP has received research reagent from AstraZeneca (no monies) and has been a site investigator for allergy and asthma clinical studies for Takeda, GlaxoSmithKline, Regeneron, Areteia, and AstraZeneca (no monies). TRM received research support from Horizon Therapeutics and has been a consultant for Horizon, Pfizer, UCB, and Sanofi.
The authors declare no competing interests.
Figures





Similar articles
-
Lung disease in relation to unique monocyte-macrophage subpopulations induced by combined inhalant endotoxin and collagen-induced arthritis.Front Immunol. 2025 Apr 9;16:1557583. doi: 10.3389/fimmu.2025.1557583. eCollection 2025. Front Immunol. 2025. PMID: 40270956 Free PMC article.
-
Aconitate decarboxylase 1 mediates the acute airway inflammatory response to environmental exposures.Front Immunol. 2024 Sep 16;15:1432334. doi: 10.3389/fimmu.2024.1432334. eCollection 2024. Front Immunol. 2024. PMID: 39351225 Free PMC article.
-
Post-endotoxin exposure-induced lung inflammation and resolution consequences beneficially impacted by lung-delivered IL-10 therapy.Sci Rep. 2022 Oct 15;12(1):17338. doi: 10.1038/s41598-022-22346-2. Sci Rep. 2022. PMID: 36243830 Free PMC article.
-
Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation.Front Biosci. 2006 May 1;11:1569-76. doi: 10.2741/1904. Front Biosci. 2006. PMID: 16368537 Review.
-
Metabolic adaption of mucosal macrophages: Is metabolism a driver of persistence across tissues?Mucosal Immunol. 2023 Oct;16(5):753-763. doi: 10.1016/j.mucimm.2023.06.006. Epub 2023 Jul 15. Mucosal Immunol. 2023. PMID: 37385586 Free PMC article. Review.
Cited by
-
Lung disease in relation to unique monocyte-macrophage subpopulations induced by combined inhalant endotoxin and collagen-induced arthritis.Front Immunol. 2025 Apr 9;16:1557583. doi: 10.3389/fimmu.2025.1557583. eCollection 2025. Front Immunol. 2025. PMID: 40270956 Free PMC article.
-
Spectral immune cell profiling reveals modulations in immune cell response to repetitive inhaled organic dust exposure in a high omega-3 fatty acid mouse model.Lipids Health Dis. 2025 Jul 2;24(1):227. doi: 10.1186/s12944-025-02651-1. Lipids Health Dis. 2025. PMID: 40604979 Free PMC article.
-
Aconitate decarboxylase 1 mediates the acute airway inflammatory response to environmental exposures.Front Immunol. 2024 Sep 16;15:1432334. doi: 10.3389/fimmu.2024.1432334. eCollection 2024. Front Immunol. 2024. PMID: 39351225 Free PMC article.
-
Lung-delivered IL-10 mitigates Lung inflammation induced by repeated endotoxin exposures in male mice.Physiol Rep. 2025 Feb;13(4):e70253. doi: 10.14814/phy2.70253. Physiol Rep. 2025. PMID: 39980189 Free PMC article.
References
-
- Després V, Huffman JA, Burrows SM, Hoose C, Safatov A, Buryak G, et al. Primary biological aerosol particles in the atmosphere: a review. Tellus B Chem Phys Meteorol. 2012;64(1):15598. doi: 10.3402/tellusb.v64i0.15598. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

