In situ chemoimmunotherapy hydrogel elicits immunogenic cell death and evokes efficient antitumor immune response
- PMID: 38594751
- PMCID: PMC11005214
- DOI: 10.1186/s12967-024-05102-0
In situ chemoimmunotherapy hydrogel elicits immunogenic cell death and evokes efficient antitumor immune response
Abstract
Background: Chemoimmunotherapy has shown promising advantages of eliciting immunogenic cell death and activating anti-tumor immune responses. However, the systemic toxicity of chemotherapy and tumor immunosuppressive microenvironment limit the clinical application.
Methods: Here, an injectable sodium alginate hydrogel (ALG) loaded with nanoparticle albumin-bound-paclitaxel (Nab-PTX) and an immunostimulating agent R837 was developed for local administration. Two murine hepatocellular carcinoma and breast cancer models were established. The tumor-bearing mice received the peritumoral injection of R837/Nab-PTX/ALG once a week for two weeks. The antitumor efficacy, the immune response, and the tumor microenvironment were investigated.
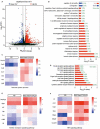
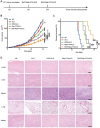
Results: This chemoimmunotherapy hydrogel with sustained-release character was proven to have significant effects on killing tumor cells and inhibiting tumor growth. Peritumoral injection of our hydrogel caused little harm to normal organs and triggered a potent antitumor immune response against both hepatocellular carcinoma and breast cancer. In the tumor microenvironment, enhanced immunogenic cell death induced by the combination of Nab-PTX and R837 resulted in 3.30-fold infiltration of effector memory T cells and upregulation of 20 biological processes related to immune responses.
Conclusions: Our strategy provides a novel insight into the combination of chemotherapy and immunotherapy and has the potential for clinical translation.
Keywords: Chemoimmunotherapy; Hydrogel; In situ vaccine; Nab-PTX; TLR7 agonist.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Nanomicelle protects the immune activation effects of Paclitaxel and sensitizes tumors to anti-PD-1 Immunotherapy.Theranostics. 2020 Jul 9;10(18):8382-8399. doi: 10.7150/thno.45391. eCollection 2020. Theranostics. 2020. PMID: 32724476 Free PMC article.
-
Ultrasound-triggered drug-loaded nanobubbles for enhanced T cell recruitment in cancer chemoimmunotherapy.Biomaterials. 2025 Jun;317:123086. doi: 10.1016/j.biomaterials.2025.123086. Epub 2025 Jan 2. Biomaterials. 2025. PMID: 39805187
-
Injectable, Adhesive Albumin Nanoparticle-Incorporated Hydrogel for Sustained Localized Drug Delivery and Efficient Tumor Treatment.ACS Appl Mater Interfaces. 2024 Feb 28;16(8):9868-9879. doi: 10.1021/acsami.3c18306. Epub 2024 Feb 13. ACS Appl Mater Interfaces. 2024. PMID: 38349713
-
Injectable hydrogels for personalized cancer immunotherapies.Acta Biomater. 2023 Dec;172:67-91. doi: 10.1016/j.actbio.2023.10.002. Epub 2023 Oct 6. Acta Biomater. 2023. PMID: 37806376 Review.
-
Tumor Microenvironment-Activatable Prodrug Vesicles for Nanoenabled Cancer Chemoimmunotherapy Combining Immunogenic Cell Death Induction and CD47 Blockade.Adv Mater. 2019 Apr;31(14):e1805888. doi: 10.1002/adma.201805888. Epub 2019 Feb 14. Adv Mater. 2019. PMID: 30762908 Review.
Cited by
-
Chemoimmunotherapy synergism: mechanisms and clinical applications.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 12. doi: 10.1007/s00210-025-04125-8. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40220027 Review.
-
Encapsulation in Alginates Hydrogels and Controlled Release: An Overview.Molecules. 2024 May 26;29(11):2515. doi: 10.3390/molecules29112515. Molecules. 2024. PMID: 38893391 Free PMC article. Review.
-
Eliciting antitumor immunity via therapeutic cancer vaccines.Cell Mol Immunol. 2025 Aug;22(8):840-868. doi: 10.1038/s41423-025-01316-4. Epub 2025 Jul 9. Cell Mol Immunol. 2025. PMID: 40629076 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

