Opicapone to Treat Early Wearing-off in Parkinson's Disease Patients: The Korean ADOPTION Trial
- PMID: 38594812
- PMCID: PMC11145137
- DOI: 10.1002/mdc3.14030
Opicapone to Treat Early Wearing-off in Parkinson's Disease Patients: The Korean ADOPTION Trial
Abstract
Background: Increasing levodopa (L-dopa)/dopa decarboxylase inhibitor (DDCI) daily dose or adding a catechol-O-methyltransferase (COMT) inhibitor to levodopa/DDCI therapy are strategies used to manage wearing-off symptoms in Parkinson's disease (PD) patients.
Objectives: To evaluate the COMT inhibitor opicapone versus an additional dose of levodopa to treat early wearing-off in PD patients.
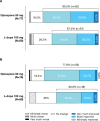
Methods: ADOPTION was a randomized, parallel-group, open-label, Phase 4 study conducted in Korea. At baseline, eligible patients were randomized (1:1) to opicapone 50 mg (n = 87) or L-dopa 100 mg (n = 81) (added to current L-dopa/DDCI therapy) for 4 weeks. The main efficacy endpoint was change from baseline to end of study in absolute off time. Other endpoints included changes in on time, in Movement Disorder Society-Unified Parkinson's Disease Rating Scale and 8-item PD Questionnaire scores, and the Clinical and Patient Global Impression of Improvement/Change.
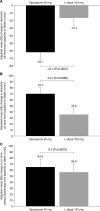
Results: The adjusted mean in absolute off time was significantly greater for opicapone 50 mg than for L-dopa 100 mg (-62.1 vs. -16.7 minutes; P = 0.0015). Opicapone-treated patients also reported a greater reduction in the percentage of off time (P = 0.0015), a greater increase in absolute on time (P = 0.0338) and a greater increase in the percentage of on time (P = 0.0015). There were no significant differences in other secondary endpoints. The L-dopa equivalent daily dose was significantly higher in the opicapone group (750.9 vs. 690.0 mg; P = 0.0247), when a 0.5 conversion factor is applied.
Conclusions: Opicapone 50 mg was more effective than an additional 100 mg L-dopa dose at decreasing off time in patients with PD and early wearing-off.
Keywords: Parkinson's disease; levodopa; opicapone; wearing off.
© 2024 The Authors. Movement Disorders Clinical Practice published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures
References
-
- Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson's disease? Eur Neurol 2010;63(5):257–266. - PubMed
-
- Prange S, Danaila T, Laurencin C, et al. Age and time course of long‐term motor and nonmotor complications in Parkinson disease. Neurology 2019;92(2):e148–e160. - PubMed
-
- Antonini A, Martinez‐Martin P, Chaudhuri RK, et al. Wearing‐off scales in Parkinson's disease: critique and recommendations. Mov Disord 2011;26(12):2169–2175. - PubMed
-
- Olanow CW, Obeso JA, Stocchi F. Continuous dopamine‐receptor treatment of Parkinson's disease: scientifi c rationale and clinical implications. Lancet Neurol 2006;5:677–687. - PubMed
-
- Olanow CW, Calabrese P, Obeso JA. Continuous dopaminergic stimulation as a treatment for Parkinson's disease: current status and future opportunities. Mov Disord 2020;35(10):1731–1744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

