Analysis of Vascular Smooth Muscle Cells from Thoracic Aortic Aneurysms Reveals DNA Damage and Cell Cycle Arrest as Hallmarks in Bicuspid Aortic Valve Patients
- PMID: 38594816
- PMCID: PMC11301675
- DOI: 10.1021/acs.jproteome.3c00649
Analysis of Vascular Smooth Muscle Cells from Thoracic Aortic Aneurysms Reveals DNA Damage and Cell Cycle Arrest as Hallmarks in Bicuspid Aortic Valve Patients
Abstract
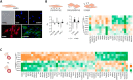
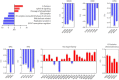
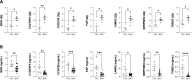
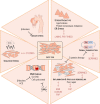
Thoracic aortic aneurysm (TAA) is mainly sporadic and with higher incidence in the presence of a bicuspid aortic valve (BAV) for unknown reasons. The lack of drug therapy to delay TAA progression lies in the limited knowledge of pathophysiology. We aimed to identify the molecular hallmarks that differentiate the aortic dilatation associated with BAV and tricuspid aortic valve (TAV). Aortic vascular smooth muscle cells (VSMCs) isolated from sporadic TAA patients with BAV or TAV were analyzed by mass spectrometry. DNA oxidative damage assay and cell cycle profiling were performed in three independent cohorts supporting proteomics data. The alteration of secreted proteins was confirmed in plasma. Stress phenotype, oxidative stress, and enhanced DNA damage response (increased S-phase arrest and apoptosis) were found in BAV-TAA patients. The increased levels of plasma C1QTNF5, LAMA2, THSB3, and FAP confirm the enhanced stress in BAV-TAA. Plasma FAP and BGN point to an increased inflammatory condition in TAV. The arterial wall of BAV patients shows a limited capacity to counteract drivers of sporadic TAA. The molecular pathways identified support the need of differential molecular diagnosis and therapeutic approaches for BAV and TAV patients, showing specific markers in plasma which may serve to monitor therapy efficacy.
Keywords: DNA damage; aortic aneurysm; bicuspid aortic valve; cardiovascular disease; oxidative stress; proteomics; vascular smooth muscle cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

