Targeting novel regulated cell death: Ferroptosis, pyroptosis and necroptosis in anti-PD-1/PD-L1 cancer immunotherapy
- PMID: 38594879
- PMCID: PMC11294428
- DOI: 10.1111/cpr.13644
Targeting novel regulated cell death: Ferroptosis, pyroptosis and necroptosis in anti-PD-1/PD-L1 cancer immunotherapy
Abstract
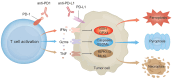
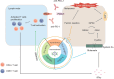
Chemotherapy, radiotherapy, and immunotherapy represent key tumour treatment strategies. Notably, immune checkpoint inhibitors (ICIs), particularly anti-programmed cell death 1 (PD1) and anti-programmed cell death ligand 1 (PD-L1), have shown clinical efficacy in clinical tumour immunotherapy. However, the limited effectiveness of ICIs is evident due to many cancers exhibiting poor responses to this treatment. An emerging avenue involves triggering non-apoptotic regulated cell death (RCD), a significant mechanism driving cancer cell death in diverse cancer treatments. Recent research demonstrates that combining RCD inducers with ICIs significantly enhances their antitumor efficacy across various cancer types. The use of anti-PD-1/PD-L1 immunotherapy activates CD8+ T cells, prompting the initiation of novel RCD forms, such as ferroptosis, pyroptosis, and necroptosis. However, the functions and mechanisms of non-apoptotic RCD in anti-PD1/PD-L1 therapy remain insufficiently explored. This review summarises the emerging roles of ferroptosis, pyroptosis, and necroptosis in anti-PD1/PD-L1 immunotherapy. It emphasises the synergy between nanomaterials and PD-1/PD-L1 inhibitors to induce non-apoptotic RCD in different cancer types. Furthermore, targeting cell death signalling pathways in combination with anti-PD1/PD-L1 therapies holds promise as a prospective immunotherapy strategy for tumour treatment.
© 2024 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ferroptosis, necroptosis, and pyroptosis in anticancer immunity.J Hematol Oncol. 2020 Aug 10;13(1):110. doi: 10.1186/s13045-020-00946-7. J Hematol Oncol. 2020. PMID: 32778143 Free PMC article. Review.
-
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy.Signal Transduct Target Ther. 2022 Jun 20;7(1):196. doi: 10.1038/s41392-022-01046-3. Signal Transduct Target Ther. 2022. PMID: 35725836 Free PMC article. Review.
-
Ferroptosis, pyroptosis and necroptosis in hepatocellular carcinoma immunotherapy: Mechanisms and immunologic landscape (Review).Int J Oncol. 2024 Jun;64(6):63. doi: 10.3892/ijo.2024.5651. Epub 2024 May 17. Int J Oncol. 2024. PMID: 38757345 Free PMC article. Review.
-
Modulation of N-glycosylation in the PD-1: PD-L1 axis as a strategy to enhance cancer immunotherapies.Biochim Biophys Acta Rev Cancer. 2025 Apr;1880(2):189274. doi: 10.1016/j.bbcan.2025.189274. Epub 2025 Jan 26. Biochim Biophys Acta Rev Cancer. 2025. PMID: 39875060 Review.
-
Exploring immune checkpoint inhibitors: Focus on PD-1/PD-L1 axis and beyond.Pathol Res Pract. 2025 May;269:155864. doi: 10.1016/j.prp.2025.155864. Epub 2025 Mar 1. Pathol Res Pract. 2025. PMID: 40068282 Review.
Cited by
-
Induced Necroptosis and Its Role in Cancer Immunotherapy.Int J Mol Sci. 2024 Oct 6;25(19):10760. doi: 10.3390/ijms251910760. Int J Mol Sci. 2024. PMID: 39409087 Free PMC article. Review.
-
CASC8 activates the pentose phosphate pathway to inhibit disulfidptosis in pancreatic ductal adenocarcinoma though the c-Myc-GLUT1 axis.J Exp Clin Cancer Res. 2025 Jan 27;44(1):26. doi: 10.1186/s13046-025-03295-w. J Exp Clin Cancer Res. 2025. PMID: 39865281 Free PMC article.
-
Exploring cell death pathways in oral cancer: mechanisms, therapeutic strategies, and future perspectives.Discov Oncol. 2025 Mar 26;16(1):395. doi: 10.1007/s12672-025-02022-3. Discov Oncol. 2025. PMID: 40133563 Free PMC article. Review.
-
Nano-immunotherapy: Merging immunotherapy precision with nanomaterial delivery.iScience. 2025 Mar 30;28(5):112319. doi: 10.1016/j.isci.2025.112319. eCollection 2025 May 16. iScience. 2025. PMID: 40292310 Free PMC article. Review.
-
Long non-coding RNAs in ferroptosis, pyroptosis and necroptosis: from functions to clinical implications in cancer therapy.Front Oncol. 2024 Aug 29;14:1437698. doi: 10.3389/fonc.2024.1437698. eCollection 2024. Front Oncol. 2024. PMID: 39267831 Free PMC article. Review.
References
-
- Desai AP, Adashek JJ, Reuss JE, West HJ, Mansfield AS. Perioperative immune checkpoint inhibition in early‐stage non‐small cell lung cancer: a review. JAMA Oncol. 2023;9:135‐142. - PubMed
-
- Emiloju OE, Sinicrope FA. Neoadjuvant immune checkpoint inhibitor therapy for localized deficient mismatch repair colorectal cancer: a review. JAMA Oncol. 2023;9:1708‐1715. - PubMed
-
- Upadhaya S, Neftelinov ST, Hodge J, Campbell J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. 2022;21:482‐483. - PubMed
-
- Gupta B, Sadaria D, Warrier VU, et al. Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy. Semin Cancer Biol. 2022;80:87‐106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

