Voluntary Wheel Running Reduces Cardiometabolic Risks in Female Offspring Exposed to Lifelong High-Fat, High-Sucrose Diet
- PMID: 38595204
- PMCID: PMC11250925
- DOI: 10.1249/MSS.0000000000003443
Voluntary Wheel Running Reduces Cardiometabolic Risks in Female Offspring Exposed to Lifelong High-Fat, High-Sucrose Diet
Abstract
Purpose: Maternal and postnatal overnutrition has been linked to an increased risk of cardiometabolic diseases in offspring. This study investigated the impact of adult-onset voluntary wheel running to counteract cardiometabolic risks in female offspring exposed to a life-long high-fat, high-sucrose (HFHS) diet.
Methods: Dams were fed either an HFHS or a low-fat, low-sucrose (LFLS) diet starting from 8 wk before pregnancy and continuing throughout gestation and lactation. Offspring followed their mothers' diets. At 15 wk of age, they were divided into sedentary (Sed) or voluntary wheel running (Ex) groups, resulting in four groups: LFLS/Sed ( n = 10), LFLS/Ex ( n = 5), HFHS/Sed ( n = 6), HFHS/Ex ( n = 5). Cardiac function was assessed at 25 wk, with tissue collection at 26 wk for mitochondrial respiratory function and protein analysis. Data were analyzed using two-way ANOVA.
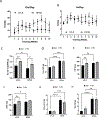
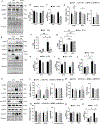
Results: Although maternal HFHS diet did not affect the offspring's body weight at weaning, continuous HFHS feeding postweaning resulted in increased body weight and adiposity, irrespective of the exercise regimen. HFHS/Sed offspring showed increased left ventricular wall thickness and elevated expression of enzymes involved in fatty acid transport (CD36, FABP3), lipogenesis (DGAT), glucose transport (GLUT4), oxidative stress (protein carbonyls, nitrotyrosine), and early senescence markers (p16, p21). Their cardiac mitochondria displayed lower oxidative phosphorylation (OXPHOS) efficiency and reduced expression of OXPHOS complexes and fatty acid metabolism enzymes (ACSL5, CPT1B). However, HFHS/Ex offspring mitigated these effects, aligning more with LFLS/Sed offspring.
Conclusions: Adult-onset voluntary wheel running effectively counteracts the detrimental cardiac effects of a lifelong HFHS diet, improving mitochondrial efficiency, reducing oxidative stress, and preventing early senescence. This underscores the significant role of physical activity in mitigating diet-induced cardiometabolic risks.
Copyright © 2024 by the American College of Sports Medicine.
Figures




Similar articles
-
Lifelong high-fat, high-sucrose diet causes sex-specific heart dysfunction in mouse offspring.Acad Med (San Franc). 2025;2(3):10.20935/acadmed7821. doi: 10.20935/acadmed7821. Epub 2025 Jul 24. Acad Med (San Franc). 2025. PMID: 40799296 Free PMC article.
-
Supplementation of the maternal diet during pregnancy with chocolate and fructose interacts with the high-fat diet of the young to facilitate the onset of metabolic disorders in rat offspring.Clin Exp Pharmacol Physiol. 2013 Sep;40(9):652-61. doi: 10.1111/1440-1681.12147. Clin Exp Pharmacol Physiol. 2013. PMID: 23819696
-
Maternal nitrate supplementation improves offspring cardiometabolic outcomes in obese pregnancies.Ann Med. 2025 Dec;57(1):2521440. doi: 10.1080/07853890.2025.2521440. Epub 2025 Jul 7. Ann Med. 2025. PMID: 40624890 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
Cited by
-
Reevaluating Anti-Inflammatory Therapy: Targeting Senescence to Balance Anti-Cancer Efficacy and Vascular Disease.Arterioscler Thromb Vasc Biol. 2025 Mar;45(3):372-385. doi: 10.1161/ATVBAHA.124.319870. Epub 2025 Jan 16. Arterioscler Thromb Vasc Biol. 2025. PMID: 39817327 Review.
References
-
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. - PubMed
-
- Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes. 2015;39(4):677–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

