Photoinduced radical tandem annulation of 1,7-diynes: an approach for divergent assembly of functionalized quinolin-2(1H)-ones
- PMID: 38595704
- PMCID: PMC11002210
- DOI: 10.3389/fchem.2024.1371978
Photoinduced radical tandem annulation of 1,7-diynes: an approach for divergent assembly of functionalized quinolin-2(1H)-ones
Abstract
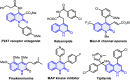
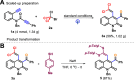
The first photocatalytic trichloromethyl radical-triggered annulative reactions of amide-linked 1,7-diynes with polyhalomethanes were established for the flexible assembly of functionalized quinolin-2(1H)-ones with generally acceptable yields. With the installation of the aryl group (R1) into the alkynyl moiety, C-center radical-initiated Kharasch-type addition/nucleophilic substitution/elimination cascade to produce quinolin-2(1H)-ones-incorporating gem-dihaloalkene, whereas three examples of polyhalogenated quinolin-2(1H)-ones were afforded when amide-linked 1,7-diynes bearing two terminal alkyne units were subjected to BrCX3 by exploiting dry acetonitrile as a solvent.
Keywords: 1,7-diynes; Kharasch addition; annulative reactions; photoinduced; quinolin-2(1H)-ones.
Copyright © 2024 Chen, Song, Yan, Li, Wang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bach T., Bergmann H., Grosch B., Harms K. (2002). Highly enantioselective intra- and intermolecular [2 + 2] photocycloaddition reactions of 2-quinolones mediated by a chiral lactam host: host-guest interactions, product configuration, and the origin of the stereoselectivity in solution. J. Am. Chem. Soc. 124, 7982–7990. 10.1021/ja0122288 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

