Elderly patient with unresectable advanced‑stage hepatocellular carcinoma who received atezolizumab plus bevacizumab and achieved a complete response: A case report
- PMID: 38595809
- PMCID: PMC11002835
- DOI: 10.3892/mi.2024.147
Elderly patient with unresectable advanced‑stage hepatocellular carcinoma who received atezolizumab plus bevacizumab and achieved a complete response: A case report
Abstract
Hepatocellular carcinoma (HCC) is a common malignancy with a poor prognosis, particularly in patients with advanced-stage disease, elderly individuals and/or in those with poor liver function. Immune checkpoint inhibitor-containing therapies, such as atezolizumab, an anti-programmed death ligand-1 monoclonal antibody, plus bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, may be effective and safe therapeutic options for elderly patients with advanced-stage HCC. The present study reports the case of a male patient his 80s who consumed alcohol with unresectable advanced-stage HCC who received combination therapy comprising atezolizumab plus bevacizumab for 6 months. The patient achieved a complete response despite the discontinuation of treatment due to nephrotoxicity. It is critical for patients with HCC and a Child-Pugh A grade to continue therapy for HCC, even if they are older. The development of more effective therapies is required for patients with advanced-stage HCC with a worse liver function than those with a Child-Pugh A grade. The case described in the present study demonstrates the need for obtaining further evidence regarding the efficacy and safety of the combination therapy including atezolizumab plus bevacizumab for elderly patients with advanced-stage HCC.
Keywords: complete response; liver cancer; older patient; stage IV disease; systemic therapy.
Copyright: © 2024 Arima et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
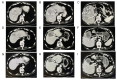
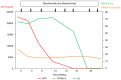
References
-
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. - DOI - PubMed
-
- Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
