Decreasing the steroid rapidly may help to improve the clinical outcomes of patients with intestinal steroid-refractory acute graft-versus-host disease receiving basiliximab treatment
- PMID: 38595816
- PMCID: PMC11002247
- DOI: 10.3389/fonc.2024.1390438
Decreasing the steroid rapidly may help to improve the clinical outcomes of patients with intestinal steroid-refractory acute graft-versus-host disease receiving basiliximab treatment
Abstract
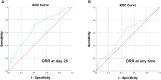
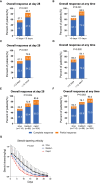
Intestinal steroid refractory acute graft-versus-host disease (SR-aGVHD) is the major cause of mortality in allogeneic hematopoietic stem cell transplantation (allo-HSCT). This retrospective cohort study aimed to identify the relationship between different steroid decreasing velocity and therapeutic response in patients with intestinal SR-aGVHD receiving basiliximab treatment, and also aimed to propose a reasonable steroid decreasing regimen for these patients. The median time for steroid dose decreasing to the 50% of initial dose and decreasing to the low-dose steroid for patients achieving ORR was 5 days and 12 days, respectively, which was both shorter than patients without achieving ORR. The ORR, NRM and survival in rapid and medium steroid decreasing group were all better than slow group. The cumulative incidence of ORR at any time was 90.4%, 78.1% and 62.3%, respectively, in rapid, medium, and slow group. The cumulative incidence of NRM at 1 year after basiliximab treatment was 18.7% (95% CI 11.3%-26.1%), 22.8% (95% CI 14.2%-31.4%) and 32.8% (95% CI 24.1%-41.5%), respectively, in rapid, medium, and slow group. The probability of OS at 1 year after basiliximab treatment was 76.9% (95% CI 68.9%-84.9%), 72.7% (95% CI 63.7%-81.7%), and 62.3% (95% CI 53.5%-71.1%), respectively, in rapid, medium, and slow group. Hence, it was helpful to decrease steroid to the 50% of initial dose ≤ 5 days and to the low-dose steroid ≤ 12 days after basiliximab treatment for intestinal SR-aGVHD patients, which may also be the reasonable steroid decrease protocol for these patients.
Keywords: acute graft-versus-host disease; basiliximab; hematopoietic stem cell transplantation; steroid decrease protocol; steroid refractory.
Copyright © 2024 Cheng, Deng, Zhang, Xu, Wang, Yan, Chen, Chen, Han, Wang, Wang, Sun, Huang and Mo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. . The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol. (2018) 11:33. doi: 10.1186/s13045-018-0564-x - DOI - PMC - PubMed
-
- Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. . The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. (2021) 14:145. doi: 10.1186/s13045-021-01159-2 - DOI - PMC - PubMed
-
- Cao Y ZC, Cao L, Mo X, Hu X. How to treat the newly diagnosed patients with FLT3-internaltandem-duplication-positive AML? Innovation Med. (2023) 1(1):100007. doi: 10.59717/j.xinn-med.2023.100007 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

