Clinical utility of plasma Aβ42/40 ratio by LC-MS/MS in Alzheimer's disease assessment
- PMID: 38595851
- PMCID: PMC11003272
- DOI: 10.3389/fneur.2024.1364658
Clinical utility of plasma Aβ42/40 ratio by LC-MS/MS in Alzheimer's disease assessment
Abstract
Introduction: Plasma Aβ42/40 ratio can help predict amyloid PET status, but its clinical utility in Alzheimer's disease (AD) assessment is unclear.
Methods: Aβ42/40 ratio was measured by LC-MS/MS for 250 specimens with associated amyloid PET imaging, diagnosis, and demographic data, and for 6,192 consecutive clinical specimens submitted for Aβ42/40 testing.
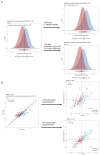
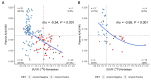
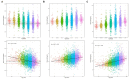
Results: High diagnostic sensitivity and negative predictive value (NPV) for Aβ-PET positivity were observed, consistent with the clinical performance of other plasma LC-MS/MS assays, but with greater separation between Aβ42/40 values for individuals with positive vs. negative Aβ-PET results. Assuming a moderate prevalence of Aβ-PET positivity, a cutpoint was identified with 99% NPV, which could help predict that AD is likely not the cause of patients' cognitive impairment and help reduce PET evaluation by about 40%.
Conclusion: High-throughput plasma Aβ42/40 LC-MS/MS assays can help identify patients with low likelihood of AD pathology, which can reduce PET evaluations, allowing for cost savings.
Keywords: Alzheimer’s disease; LC-MS/MS; PET; beta-amyloid; blood biomarkers; prescreening.
Copyright © 2024 Weber, Taylor, Lagier, Kim, Goldman, Clarke, Vaillancourt, Duara, McFarland, Wang, Golde and Racke.
Conflict of interest statement
DW, ST, RL, JK, SG, NC, and MR were employees of Quest Diagnostics. DW and NC hold patents for the detection of beta-amyloid by mass spectrometry as well as for the detection of apolipoprotein E proteoforms by mass spectrometry. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Update of
-
Clinical utility of plasma Aβ42/40 ratio by LC-MS/MS in Alzheimer's disease assessment.medRxiv [Preprint]. 2023 Dec 14:2023.12.12.23299878. doi: 10.1101/2023.12.12.23299878. medRxiv. 2023. Update in: Front Neurol. 2024 Mar 25;15:1364658. doi: 10.3389/fneur.2024.1364658. PMID: 38168329 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

