Dosing optimization of rituximab for primary membranous nephropathy by population pharmacokinetic and pharmacodynamic study
- PMID: 38595918
- PMCID: PMC11002205
- DOI: 10.3389/fphar.2024.1197651
Dosing optimization of rituximab for primary membranous nephropathy by population pharmacokinetic and pharmacodynamic study
Abstract
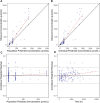
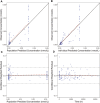
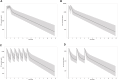
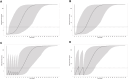
Primary membranous nephropathy (PMN) is the most common cause for adult nephrotic syndrome. Rituximab has demonstrated promising clinical efficacy by random controlled trials and the off-label use is widely adopted in PMN. However, the standard dosage is borrowed from B cell lymphoma treatment with far more antigens and is oversaturated for PMN treatment, accompanied with additional safety risk and unnecessary medical cost. More than 15% serious adverse events were observed under standard dosage and low dose therapies were explored recently. Dose optimization by clinical trials is extremely time- and cost-consuming and can be significantly accelerated with the aid of model-informed drug development. Here, we aim to establish the first population pharmacokinetic and pharmacodynamic (PPK/PD) model for rituximab in PMN to guide its dosage optimization. Rituximab pharmacokinetic and pharmacodynamic data from 41 PMN patients in a retrospective study under a newly proposed monthly mini-dose were used to construct quantitative dose-exposure-response relationship via mechanistic target-mediated drug disposition (TMDD) model followed by regression between the reduction of anti-PLA2R titer and time after the treatment. The final model, validated by goodness-of-fit plots, visual predictive checks and bootstrap, was used to recommend the optimized dosing regimen by simulations. The model was well validated for PK/PD prediction. The systemic clearance and half-life are 0.54 L/h and 14.7 days, respectively. Simulation of a novel regimen (6 monthly doses of 100 mg) indicated the comparable ability and superior duration time of CD20+ B cell depletion compared with standard dosage, while the cumulative dosage and safety risk was significantly decreased. We established the first PPK/PD model and provide evidence to support the dosage optimization based on monthly mini-dose. Our study can also efficiently accelerate dosage optimization of novel anti-CD20 antibodies in PMN and other indications.
Keywords: dosage optimization; population pharmacokinetic and pharmacodynamic; primary membranous nephropathy; rituximab; target-mediated drug disposition.
Copyright © 2024 Liang, Deng, Niu, Kong, Liu, Wang, Li, Wang, Zheng and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Different Dosage Regimens of Rituximab in Primary Membranous Nephropathy Treatment: A Systematic Review.Int J Nephrol Renovasc Dis. 2024 Oct 29;17:265-273. doi: 10.2147/IJNRD.S489455. eCollection 2024. Int J Nephrol Renovasc Dis. 2024. PMID: 39493295 Free PMC article. Review.
-
Monthly mini-dose rituximab for primary anti-PLA2R-positive membranous nephropathy: a personalized approach.BMC Nephrol. 2023 May 26;24(1):146. doi: 10.1186/s12882-023-03206-1. BMC Nephrol. 2023. PMID: 37237260 Free PMC article.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Low-dose rituximab is poorly effective in patients with primary membranous nephropathy.Nephrol Dial Transplant. 2017 Oct 1;32(10):1691-1696. doi: 10.1093/ndt/gfw251. Nephrol Dial Transplant. 2017. PMID: 27387472
-
Efficacy and safety of rituximab for primary membranous nephropathy with different clinical presentations: a retrospective study.Front Immunol. 2023 Apr 28;14:1156470. doi: 10.3389/fimmu.2023.1156470. eCollection 2023. Front Immunol. 2023. PMID: 37187749 Free PMC article.
Cited by
-
Different Dosage Regimens of Rituximab in Primary Membranous Nephropathy Treatment: A Systematic Review.Int J Nephrol Renovasc Dis. 2024 Oct 29;17:265-273. doi: 10.2147/IJNRD.S489455. eCollection 2024. Int J Nephrol Renovasc Dis. 2024. PMID: 39493295 Free PMC article. Review.
-
Kinetic-pharmacodynamic model to predict post-rituximab B-cell repletion as a predictor of relapse in pediatric idiopathic nephrotic syndrome.Front Pharmacol. 2025 Jan 7;15:1526936. doi: 10.3389/fphar.2024.1526936. eCollection 2024. Front Pharmacol. 2025. PMID: 39840094 Free PMC article.
-
Telitacicept monotherapy for refractory idiopathic membranous nephropathy: a case report and literature review.Front Med (Lausanne). 2025 Apr 8;12:1571616. doi: 10.3389/fmed.2025.1571616. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40265191 Free PMC article.
-
Does one model fit all mAbs? An evaluation of population pharmacokinetic models.MAbs. 2025 Dec;17(1):2512217. doi: 10.1080/19420862.2025.2512217. Epub 2025 May 30. MAbs. 2025. PMID: 40447562 Free PMC article.
References
-
- Bensalem A., Mulleman D., Paintaud G., Azzopardi N., Gouilleux-Gruart V., Cornec D., et al. (2020). Non-linear rituximab pharmacokinetics and complex relationship between rituximab concentrations and anti-neutrophil cytoplasmic antibodies (ANCA) in ANCA-associated vasculitis: the RAVE trial revisited. Clin. Pharmacokinet. 59 (4), 519–530. 10.1007/s40262-019-00826-5 - DOI - PubMed
LinkOut - more resources
Full Text Sources

