Conditionally replicative adenovirus as a therapy for malignant peripheral nerve sheath tumors
- PMID: 38595983
- PMCID: PMC10959710
- DOI: 10.1016/j.omton.2024.200783
Conditionally replicative adenovirus as a therapy for malignant peripheral nerve sheath tumors
Abstract
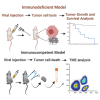
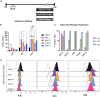
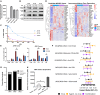
Oncolytic adenoviruses (Ads) stand out as a promising strategy for the targeted infection and lysis of tumor cells, with well-established clinical utility across various malignancies. This study delves into the therapeutic potential of oncolytic Ads in the context of neurofibromatosis type 1 (NF1)-associated malignant peripheral nerve sheath tumors (MPNSTs). Specifically, we evaluate conditionally replicative adenoviruses (CRAds) driven by the cyclooxygenase 2 (COX2) promoter, as selective agents against MPNSTs, demonstrating their preferential targeting of MPNST cells compared with non-malignant Schwann cell control. COX2-driven CRAds, particularly those with modified fiber-knobs exhibit superior binding affinity toward MPNST cells and demonstrate efficient and preferential replication and lysis of MPNST cells, with minimal impact on non-malignant control cells. In vivo experiments involving intratumoral CRAd injections in immunocompromised mice with human MPNST xenografts significantly extend survival and reduce tumor growth rate compared with controls. Moreover, in immunocompetent mouse models with MPNST-like allografts, CRAd injections induce a robust infiltration of CD8+ T cells into the tumor microenvironment (TME), indicating the potential to promote a pro-inflammatory response. These findings underscore oncolytic Ads as promising, selective, and minimally toxic agents for MPNST therapy, warranting further exploration.
Keywords: MT: Regular Issue; conditionally replicative adenovirus (CRAd); cyclooxygenase 2 (COX2); malignant peripheral nerve sheath tumor (MPNST); neurofibromatosis type 1 (NF1); oncolytic adenovirus.
Conflict of interest statement
D.A.L. is the co-founder and co-owner of NeoClone Biotechnologies, Inc., Discovery Genomics, Inc. (acquired by Immusoft, Inc.), B-MoGen Biotechnologies, Inc. (acquired by Bio-Techne corporation), and Luminary Therapeutics, Inc. D.A.L. holds equity in, is a Board of Directors member of, and serves as the Senior Scientific Advisor to Recombinetics, a genome-editing company, and Makana, a xenotransplantation company. D.A.L. consults for Styx Biotechnologies, Inc. and Genentech, Inc., which is funding some of his research. The business of all the companies above is unrelated to the contents of this manuscript.
Figures




Similar articles
-
Treatment of orthotopic malignant peripheral nerve sheath tumors with oncolytic herpes simplex virus.Neuro Oncol. 2014 Aug;16(8):1057-66. doi: 10.1093/neuonc/not317. Epub 2014 Jan 26. Neuro Oncol. 2014. PMID: 24470552 Free PMC article.
-
Malignant peripheral nerve sheath tumors with high and low Ras-GTP are permissive for oncolytic herpes simplex virus mutants.Pediatr Blood Cancer. 2006 Jun;46(7):745-54. doi: 10.1002/pbc.20565. Pediatr Blood Cancer. 2006. PMID: 16124003
-
Patterns of recurrence and survival in sporadic, neurofibromatosis Type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors.J Neurosurg. 2017 Jan;126(1):319-329. doi: 10.3171/2015.12.JNS152443. Epub 2016 Apr 1. J Neurosurg. 2017. PMID: 27035165 Free PMC article.
-
Non-cytotoxic systemic treatment in malignant peripheral nerve sheath tumors (MPNST): A systematic review from bench to bedside.Crit Rev Oncol Hematol. 2019 Jun;138:223-232. doi: 10.1016/j.critrevonc.2019.04.007. Epub 2019 Apr 19. Crit Rev Oncol Hematol. 2019. PMID: 31092379
-
New Model Systems and the Development of Targeted Therapies for the Treatment of Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors.Genes (Basel). 2020 Apr 28;11(5):477. doi: 10.3390/genes11050477. Genes (Basel). 2020. PMID: 32353955 Free PMC article. Review.
References
-
- Le Guellec S., Decouvelaere A.V., Filleron T., Valo I., Charon-Barra C., Robin Y.M., Terrier P., Chevreau C., Coindre J.M. Malignant Peripheral Nerve Sheath Tumor Is a Challenging Diagnosis: A Systematic Pathology Review, Immunohistochemistry, and Molecular Analysis in 160 Patients from the French Sarcoma Group Database. Am. J. Surg. Pathol. 2016;40:896–908. doi: 10.1097/PAS.0000000000000655. - DOI - PubMed
-
- Upadhyaya M., Kluwe L., Spurlock G., Monem B., Majounie E., Mantripragada K., Ruggieri M., Chuzhanova N., Evans D.G., Ferner R., et al. Germline and Somatic NF1 Gene Mutation Spectrum in NF1-Associated Malignant Peripheral Nerve Sheath Tumors (MPNSTs) Hum. Mutat. 2008;29:74–82. doi: 10.1002/humu.20601. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
