Enteric-coating film effect on the delayed drug release of pantoprazole gastro-resistant generic tablets
- PMID: 38596002
- PMCID: PMC11002526
- DOI: 10.12688/f1000research.140607.1
Enteric-coating film effect on the delayed drug release of pantoprazole gastro-resistant generic tablets
Abstract
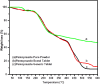
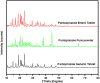
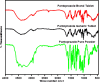
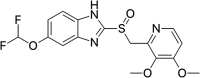
Background: Enteric coating films in acidic labile tablets protect the drug molecule from the acidic environment of the stomach. However, variations in the excipients used in the coating formulation may affect their ability to provide adequate protection. This study is the first to investigate the potential effects of coating materials on the protective functionality of enteric coating films for pantoprazole (PNZ) generic tablets after their recall from the market. Methods: A comparative analysis was conducted between generic and branded PNZ products, using pure drug powder for identification. The in vitro release of the drug was evaluated in different pH media. The study also utilized various analytical and thermal techniques, including differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared (FTIR), and confocal Raman microscopy. Results: The in vitro assessment results revealed significant variations in the release profile for the generic product in acidic media at 120 min. DSC and TGA thermal profile analyses showed slight variation between the two products. XRD analysis exhibited a noticeable difference in peak intensity for the generic sample, while SEM revealed smaller particle sizes in the generic product. The obtained spectra profile for the generic product displayed significant variation in peaks and band intensity, possibly due to impurities. These findings suggest that the excipients used in the enteric coating film of the generic product may have affected its protective functionality, leading to premature drug release in acidic media. Additionally, the presence of polysorbate 80 (P-80) in the brand product might improve the properties of the enteric coating film due to its multi-functionality. Conclusions: In conclusion, the excipients used in the brand product demonstrated superior functionality in effectively protecting the drug molecule from acidic media through the enteric coating film, as compared to the generic version.
Keywords: Enteric Coating Film; Pantoprazole; In vitro Drug Release; Analytical Techniques; Differential Scanning Calorimetry; Thermogravimetric Analysis; Generic Drug; Polysorbate 80..
Copyright: © 2023 Arafat M et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
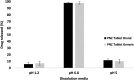




Similar articles
-
Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets.Pharmaceuticals (Basel). 2023 Apr 4;16(4):539. doi: 10.3390/ph16040539. Pharmaceuticals (Basel). 2023. PMID: 37111296 Free PMC article.
-
Alginate-magnesium aluminum silicate films: effect of plasticizers on film properties, drug permeation and drug release from coated tablets.Int J Pharm. 2007 Mar 21;333(1-2):34-44. doi: 10.1016/j.ijpharm.2006.09.046. Epub 2006 Sep 30. Int J Pharm. 2007. PMID: 17056214
-
[Preparation of pantoprazole sodium enteric-coated pellets-type tablets].Yao Xue Xue Bao. 2011 Jan;46(1):96-101. Yao Xue Xue Bao. 2011. PMID: 21465814 Chinese.
-
Development of Delayed Release Oral Formulation Comprising Esomeprazole Spray Dried Dispersion Utilizing Design of Experiment As An Optimization Strategy.AAPS PharmSciTech. 2023 Sep 12;24(7):186. doi: 10.1208/s12249-023-02642-4. AAPS PharmSciTech. 2023. PMID: 37700215
-
A Review on Enteric Coated Pellets Composed of Core Pellets Prepared by Extrusion-Spheronization.Recent Pat Drug Deliv Formul. 2019;13(2):83-90. doi: 10.2174/1872211313666190212115139. Recent Pat Drug Deliv Formul. 2019. PMID: 30747090 Review.
Cited by
-
Effect of Hydration Forms and Polymer Grades on Theophylline Controlled-Release Tablet: An Assessment and Evaluation.Pharmaceuticals (Basel). 2024 Feb 21;17(3):271. doi: 10.3390/ph17030271. Pharmaceuticals (Basel). 2024. PMID: 38543057 Free PMC article.
References
-
- Porter S, Sackett G, Liu L: Development, Optimization, and Scale-Up of Process Parameters. Developing Solid Oral Dosage Forms. Elsevier;2017; pp.953–996. 10.1016/B978-0-12-802447-8.00034-0 - DOI
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

