Quantity, distribution and phenotype of newly generated cells in the intact spinal cord of adult macaque monkeys
- PMID: 38596108
- PMCID: PMC11002253
- DOI: 10.1016/j.heliyon.2024.e28856
Quantity, distribution and phenotype of newly generated cells in the intact spinal cord of adult macaque monkeys
Abstract
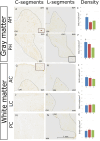
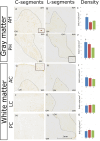
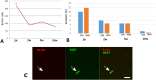
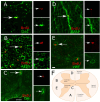
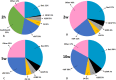
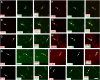
The existence of proliferating cells in the intact spinal cord, their distribution and phenotype, are well studied in rodents. A limited number of studies also address the proliferation after spinal cord injury, in non-human primates. However, a detailed description of the quantity, distribution and phenotype of proliferating cells at different anatomical levels of the intact adult non-human primate spinal cord is lacking at present. In the present study, we analyzed normal spinal cord tissues from adult macaque monkeys (Macaca fuscata), infused with Bromo-2'-deoxyuridine (BrdU), and euthanized at 2h, 2 weeks, 5 weeks and 10 weeks after BrdU. We found a significantly higher density of BrdU + cells in the gray matter of cervical segments as compared to thoracic or lumbar segments, and a significantly higher density of proliferating cells in the posterior as compared to the anterior horn of the gray matter. BrdU + cells exhibited phenotype of microglia or endothelial cells (∼50%) or astroglial and oligodendroglial cells (∼40%), including glial progenitor phenotypes marked by the transcription factors Sox9 and Sox10. BrdU + cells also co-expressed other transcription factors known for their involvement in embryonic development, including Emx2, Sox1, Sox2, Ngn1, Olig1, Olig2, Olig3. In the central canal, BrdU + cells were located along the dorso-ventral axis and co-labeled for the markers Vimentin and Nestin. These results reveal the extent of cellular plasticity in the spinal cord of non-human primates under normal conditions.
Keywords: Central canal; Proliferation; Spinal cord; Transcription factor.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Anton Tonchev reports financial support was provided by Medical University Varna Prof Dr Paraskev Stoyanov. Tetsumori Yamashima reports financial support was provided by Kanazawa University 10.13039/100022395Graduate School of Medical Sciences.
Figures


Similar articles
-
Methylprednisolone inhibits the proliferation of endogenous neural stem cells in nonhuman primates with spinal cord injury.J Neurosurg Spine. 2018 Aug;29(2):199-207. doi: 10.3171/2017.12.SPINE17669. Epub 2018 May 18. J Neurosurg Spine. 2018. PMID: 29775163
-
Compression injury in the mouse spinal cord elicits a specific proliferative response and distinct cell fate acquisition along rostro-caudal and dorso-ventral axes.Neuroscience. 2013 Dec 19;254:1-17. doi: 10.1016/j.neuroscience.2013.09.011. Epub 2013 Sep 14. Neuroscience. 2013. PMID: 24042034
-
Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord.J Neurosci. 2000 Mar 15;20(6):2218-28. doi: 10.1523/JNEUROSCI.20-06-02218.2000. J Neurosci. 2000. PMID: 10704497 Free PMC article.
-
Adult neurogenesis in primate and rodent spinal cord: comparing a cervical dorsal rhizotomy with a dorsal column transection.Eur J Neurosci. 2007 Nov;26(10):2777-94. doi: 10.1111/j.1460-9568.2007.05871.x. Eur J Neurosci. 2007. PMID: 18001275
-
Traumatic injury of the spinal cord and nitric oxide.Prog Brain Res. 2007;161:171-83. doi: 10.1016/S0079-6123(06)61011-X. Prog Brain Res. 2007. PMID: 17618976 Review.
References
-
- Morshead C.M., Reynolds B.A., Craig C.G., McBurney M.W., Staines W.A., Morassutti D., Weiss S., van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. - DOI - PubMed
-
- Chiasson B.J., Tropepe V., Morshead C.M., van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J. Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

