Empowering pancreatic tumor homing with augmented anti-tumor potency of CXCR2-tethered CAR-NK cells
- PMID: 38596297
- PMCID: PMC10926211
- DOI: 10.1016/j.omton.2024.200777
Empowering pancreatic tumor homing with augmented anti-tumor potency of CXCR2-tethered CAR-NK cells
Abstract
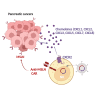
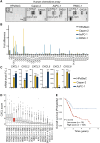
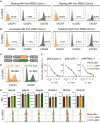
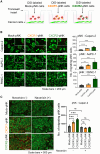
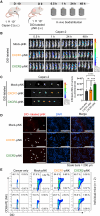
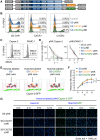
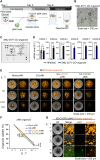
Chimeric antigen receptor (CAR)-engineered natural killer (NK) cells are a promising immunotherapy for solid cancers; however, their effectiveness against pancreatic cancer is limited by the immunosuppressive tumor microenvironment. In particular, low NK cell infiltration poses a major obstacle that reduces cytotoxicity. The current study aimed to enhance the tumor-homing capacity of CAR-NK cells by targeting the chemokine-chemokine receptor axis between NK and pancreatic cancer cells. To this end, data from a chemokine array and The Cancer Genome Atlas pan-cancer cohort were analyzed. Pancreatic cancer cells were found to secrete high levels of ligands for C-X-C motif receptor 1 (CXCR1) and CXCR2. Subsequently, we generated anti-mesothelin CAR-NK cells incorporating CXCR1 or CXCR2 and evaluated their tumor-killing abilities in 2D cancer cell co-culture and 3D tumor-mimetic organoid models. CAR-NK cells engineered with CXCR2 demonstrated enhanced tumor killing and strong infiltration of tumor sites. Collectively, these findings highlight the potential of CXCR2-augmented CAR-NK cells as a clinically relevant modality for effective pancreatic cancer treatment. By improving their infiltration and tumor-killing capabilities, these CXCR2-augmented CAR-NK cells have the potential to overcome the challenges posed by the immunosuppressive tumor microenvironment, providing improved therapeutic outcomes.
Keywords: CAR-NK; MT: Regular Issue; NK infiltration; chemokine receptors; chemotaxis; pancreatic cancer; tumor microenvironment.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
