Mediation of antitumor activity by AZD4820 oncolytic vaccinia virus encoding IL-12
- PMID: 38596304
- PMCID: PMC10869731
- DOI: 10.1016/j.omton.2023.200758
Mediation of antitumor activity by AZD4820 oncolytic vaccinia virus encoding IL-12
Abstract
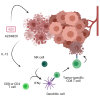
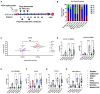
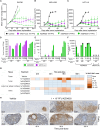
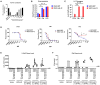
Oncolytic viruses are engineered to selectively kill tumor cells and have demonstrated promising results in early-phase clinical trials. To further modulate the innate and adaptive immune system, we generated AZD4820, a vaccinia virus engineered to express interleukin-12 (IL-12), a potent cytokine involved in the activation of natural killer (NK) and T cells and the reprogramming of the tumor immune microenvironment. Testing in cultured human tumor cell lines demonstrated broad in vitro oncolytic activity and IL-12 transgene expression. A surrogate virus expressing murine IL-12 demonstrated antitumor activity in both MC38 and CT26 mouse syngeneic tumor models that responded poorly to immune checkpoint inhibition. In both models, AZD4820 significantly upregulated interferon-gamma (IFN-γ) relative to control mice treated with oncolytic vaccinia virus (VACV)-luciferase. In the CT26 study, 6 of 10 mice had a complete response after treatment with AZD4820 murine surrogate, whereas control VACV-luciferase-treated mice had 0 of 10 complete responders. AZD4820 treatment combined with anti-PD-L1 blocking antibody augmented tumor-specific T cell immunity relative to monotherapies. These findings suggest that vaccinia virus delivery of IL-12, combined with immune checkpoint blockade, elicits antitumor immunity in tumors that respond poorly to immune checkpoint inhibitors.
Keywords: IL-12; MT: Regular Issue; oncolytic; transgene; vaccinia; virus.
© 2024 The Author(s).
Conflict of interest statement
At the time this study was conducted, C.K., S.A., A.M., E.G., S.B., A.V., R.R., K.S., N.R.M., P.S., C.B., S.A.H., E.J.K., J.L., N.M.D., M.O., and M.A.S.B. were employees of AstraZeneca, with stock ownership and/or stock options or interests in the company; and J.F., N.S., E.Q., C.D., and P.K. were employees and stockholders of Transgene SA. N.R.M is an employee of Ratio Therapeutics (Boston, MA).
Figures



References
-
- Galanis E., Hartmann L.C., Cliby W.A., Long H.J., Peethambaram P.P., Barrette B.A., Kaur J.S., Haluska P.J., Jr., Aderca I., Zollman P.J., et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. - PMC - PubMed
-
- Kaufman H.L., Kim D.W., DeRaffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg Oncol. 2010;17:718–730. - PubMed
-
- Kurokawa C., Iankov I.D., Anderson S.K., Aderca I., Leontovich A.A., Maurer M.J., Oberg A.L., Schroeder M.A., Giannini C., Greiner S.M., et al. Constitutive interferon pathway activation in tumors as an efficacy determinant following oncolytic virotherapy. J. Natl. Cancer Inst. 2018;110:1123–1132. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
