Re-evaluating efficacy of vaccines against highly pathogenic avian influenza virus in poultry: A systematic review and meta-analysis
- PMID: 38596323
- PMCID: PMC11002887
- DOI: 10.1016/j.onehlt.2024.100714
Re-evaluating efficacy of vaccines against highly pathogenic avian influenza virus in poultry: A systematic review and meta-analysis
Abstract
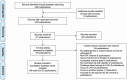
The global spread of highly pathogenic avian influenza (HPAI) A (H5N1) clade 2.3.4.4b virus since 2021 necessitates a re-evaluation of the role of vaccination in controlling HPAI outbreaks among poultry, which has been controversial because of the concern of silent spread with viral mutation and spillover to human. We systematically reviewed and meta-analyzed all existing data from experimental challenge trials to assess the efficacy of HPAI vaccines against mortality in specific pathogen free (SPF) chickens, with evaluation of the certainty of evidence (CoE) using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. Out of 223 screened publications, 46 trials met our eligibility criteria. Inactivated vaccines showed an efficacy of 95% (risk ratio [RR] = 5% [95% CI: 1% to 17%], I2 = 0%, CoE high) against homologous strains and an efficacy of 78% (RR = 22% [95% CI: 14% to 37%], I2 = 18%, CoE high) against heterologous strains (test for subgroup difference p = 0.02). Live recombinant vaccines exhibited the highest efficacy at 97% (RR = 3% [95% CI: 1% to 13%], I2 = 0%, CoE high). Inactivated recombinant vaccines had an overall efficacy of 90% (RR = 10% [95% CI: 6% to 16%], I2 = 47%, CoE high). Commercial vaccines showed an overall efficacy of 91% (RR = 9% [95% CI: 5% to 17%], I2 = 23%, CoE high), with 96% efficacy (RR = 4% [95% CI: 1% to 21%], I2 = 0%, CoE high) against homologous strains and 90% efficacy (RR = 10% [95% CI: 5% to 20%], I2 = 31%, CoE moderate) against heterologous strains. Our systematic review offers an updated and unbiased assessment of vaccine efficacy against HPAI-related mortality, providing timely and crucial information for re-evaluating the role of vaccination in poultry avian influenza control policy amist the global HPAI outbreak post-2021.
Keywords: HPAI; Highly pathogenic avian influenza; Meta-analysis; Vaccine; Vaccine efficacy.
© 2024 The Authors.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures

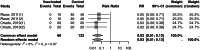


Similar articles
-
The efficacy of an inactivated avian influenza H5N1 vaccine against an African strain of HPAI H5N8 (clade 2.3.4.4 B).Avian Pathol. 2023 Jun;52(3):176-184. doi: 10.1080/03079457.2023.2181145. Epub 2023 May 2. Avian Pathol. 2023. PMID: 37079321
-
Examination of the protective efficacy of two avian influenza H5 vaccines against clade 2.3.4.4b H5N8 highly pathogenic avian influenza virus in commercial broilers.Res Vet Sci. 2021 Nov;140:125-133. doi: 10.1016/j.rvsc.2021.08.012. Epub 2021 Aug 17. Res Vet Sci. 2021. PMID: 34425414
-
Immunogenicity and Cross-Protective Efficacy Induced by an Inactivated Recombinant Avian Influenza A/H5N1 (Clade 2.3.4.4b) Vaccine against Co-Circulating Influenza A/H5Nx Viruses.Vaccines (Basel). 2023 Aug 22;11(9):1397. doi: 10.3390/vaccines11091397. Vaccines (Basel). 2023. PMID: 37766075 Free PMC article.
-
Rational approach to vaccination against highly pathogenic avian influenza in Nigeria: a scientific perspective and global best practice.Arch Virol. 2023 Sep 29;168(10):263. doi: 10.1007/s00705-023-05888-2. Arch Virol. 2023. PMID: 37775596 Review.
-
Avian Influenza Clade 2.3.4.4b: Global Impact and Summary Analysis of Vaccine Trials.Vaccines (Basel). 2025 Apr 24;13(5):453. doi: 10.3390/vaccines13050453. Vaccines (Basel). 2025. PMID: 40432065 Free PMC article. Review.
Cited by
-
Feathered fears: Could avian H5N1 influenza be the next pandemic threat of disease X?New Microbes New Infect. 2024 Apr 22;59:101416. doi: 10.1016/j.nmni.2024.101416. eCollection 2024 Jun. New Microbes New Infect. 2024. PMID: 38707625 Free PMC article. No abstract available.
-
H5N1 Avian Influenza: A Narrative Review of Scientific Advances and Global Policy Challenges.Viruses. 2025 Jun 29;17(7):927. doi: 10.3390/v17070927. Viruses. 2025. PMID: 40733545 Free PMC article. Review.
-
Research publications and global manufacture of veterinary vaccines against avian influenza A (2019-2023).Front Vet Sci. 2025 Mar 12;12:1394675. doi: 10.3389/fvets.2025.1394675. eCollection 2025. Front Vet Sci. 2025. PMID: 40144520 Free PMC article.
References
-
- Wille M., Barr I.G. Resurgence of avian influenza virus. Science. 2022;376(6592):459–460. https://www.science.org/doi/10.1126/science.abo1232. - PubMed
-
- Xie R., Edwards K.M., Wille M., Wei X., Wong S.S., Zanin M., Dhanasekaran V. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature. 2023:1–8. https://www.nature.com/articles/s41586-023-06631-2 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

