Anti-bacterial and anti-biofilm activities of arachidonic acid against the cariogenic bacterium Streptococcus mutans
- PMID: 38596377
- PMCID: PMC11002910
- DOI: 10.3389/fmicb.2024.1333274
Anti-bacterial and anti-biofilm activities of arachidonic acid against the cariogenic bacterium Streptococcus mutans
Abstract
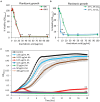
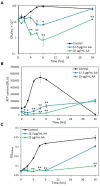
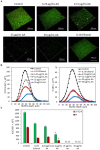
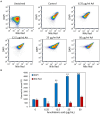
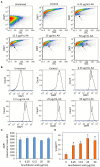
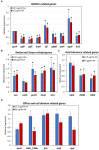
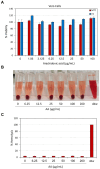
Streptococcus mutans is a Gram-positive, facultative anaerobic bacterium, which causes dental caries after forming biofilms on the tooth surface while producing organic acids that demineralize enamel and dentin. We observed that the polyunsaturated arachidonic acid (AA) (ω-6; 20:4) had an anti-bacterial activity against S. mutans, which prompted us to investigate its mechanism of action. The minimum inhibitory concentration (MIC) of AA on S. mutans was 25 μg/ml in the presence of 5% CO2, while it was reduced to 6.25-12.5 μg/ml in the absence of CO2 supplementation. The anti-bacterial action was due to a combination of bactericidal and bacteriostatic effects. The minimum biofilm inhibitory concentration (MBIC) was the same as the MIC, suggesting that part of the anti-biofilm effect was due to the anti-bacterial activity. Gene expression studies showed decreased expression of biofilm-related genes, suggesting that AA also has a specific anti-biofilm effect. Flow cytometric analyses using potentiometric DiOC2(3) dye, fluorescent efflux pump substrates, and live/dead SYTO 9/propidium iodide staining showed that AA leads to immediate membrane hyperpolarization, altered membrane transport and efflux pump activities, and increased membrane permeability with subsequent membrane perforation. High-resolution scanning electron microscopy (HR-SEM) showed remnants of burst bacteria. Furthermore, flow cytometric analysis using the redox probe 2',7'-dichlorofluorescein diacetate (DCFHDA) showed that AA acts as an antioxidant in a dose-dependent manner. α-Tocopherol, an antioxidant that terminates the radical chain, counteracted the anti-bacterial activity of AA, suggesting that oxidation of AA in bacteria leads to the production of cytotoxic radicals that contribute to bacterial growth arrest and death. Importantly, AA was not toxic to normal Vero epithelial cells even at 100 μg/ml, and it did not cause hemolysis of erythrocytes. In conclusion, our study shows that AA is a potentially safe drug that can be used to reduce the bacterial burden of cariogenic S. mutans.
Keywords: Streptococcus mutans; anti-bacterial; anti-biofilm; antioxidant; arachidonic acid; efflux pumps; membrane perforation.
Copyright © 2024 Chamlagain, Hu, Sionov and Steinberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources

