Reirradiation With Proton Therapy for Recurrent Malignancies of the Esophagus and Gastroesophageal Junction: Results of the Proton Collaborative Group Multi-Institutional Prospective Registry Trial
- PMID: 38596455
- PMCID: PMC11002543
- DOI: 10.1016/j.adro.2024.101459
Reirradiation With Proton Therapy for Recurrent Malignancies of the Esophagus and Gastroesophageal Junction: Results of the Proton Collaborative Group Multi-Institutional Prospective Registry Trial
Abstract
Purpose: Treatment options for recurrent esophageal cancer (EC) previously treated with radiation therapy (RT) are limited. Reirradiation (reRT) with proton beam therapy (PBT) can offer lower toxicities by limiting doses to surrounding tissues. In this study, we present the first multi-institutional series reporting on toxicities and outcomes after reRT for locoregionally recurrent EC with PBT.
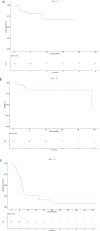
Methods and materials: Analysis of the prospective, multicenter, Proton Collaborative Group registry of patients with recurrent EC who had previously received photon-based RT and underwent PBT reRT was performed. Patient/tumor characteristics, treatment details, outcomes, and toxicities were collected. Local control (LC), distant metastasis-free survival (DMFS), and overall survival (OS) were estimated using the Kaplan-Meier method. Event time was determined from reRT start.
Results: Between 2012 and 2020, 31 patients received reRT via uniform scanning/passive scattering (61.3%) or pencil beam scanning (38.7%) PBT at 7 institutions. Median prior RT, PBT reRT, and cumulative doses were 50.4 Gy (range, 37.5-110.4), 48.6 Gy (relative biological effectiveness) (25.2-72.1), and 99.9 Gy (79.1-182.5), respectively. Of these patients, 12.9% had 2 prior RT courses, and 67.7% received PBT with concurrent chemotherapy. Median follow-up was 7.2 months (0.9-64.7). Post-PBT, there were 16.7% locoregional only, 11.1% distant only, and 16.7% locoregional and distant recurrences. Six-month LC, DMFS, and OS were 80.5%, 83.4%, and 69.1%, respectively. One-year LC, DMFS, and OS were 67.1%, 83.4%, and 27%, respectively. Acute grade ≥3 toxicities occurred in 23% of patients, with 1 acute grade 5 toxicity secondary to esophageal hemorrhage, unclear if related to reRT or disease progression. No grade ≥3 late toxicities were reported.
Conclusions: In the largest report to date of PBT for reRT in patients with recurrent EC, we observed acceptable acute toxicities and encouraging rates of disease control. However, these findings are limited by the poor prognoses of these patients, who are at high risk of mortality. Further research is needed to better assess the long-term benefits and toxicities of PBT in this specific patient population.
© 2024 The Authors.
Conflict of interest statement
J. Isabelle Choi and Charles B. Simone report personal fees from Varian Medical Systems outside of the scope of this work. Alexandra Hotca, Kunal K. Sindhu, Eric J. Lehrer, William F. Hartsell, Carlos Vargas, Henry K. Tsai, John H. Chang, Smith Apisarnthanarax, Romaine C. Nichols, Arpit M. Chhabra, Shaakir Hasan, Robert H. Press, Stanislav Lazarev, Carla Hajj, Rafi Kabarriti, and William G. Rule have no conflicts of interest to disclose.
Figures
References
-
- Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010–1021. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J Clin Oncol. 2021;39:1995–2004. - PubMed
-
- Lee PC, Mirza FM, Port JL, et al. Predictors of recurrence and disease-free survival in patients with completely resected esophageal carcinoma. J Thorac Cardiovasc Surg. 2011;141:1196–1206. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources