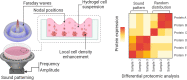
Differential proteomics profile of microcapillary networks in response to sound pattern-driven local cell density enhancement
- PMID: 38596510
- PMCID: PMC11001772
- DOI: 10.1016/j.bbiosy.2024.100094
Differential proteomics profile of microcapillary networks in response to sound pattern-driven local cell density enhancement
Abstract
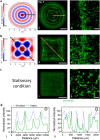
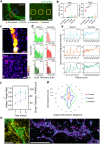
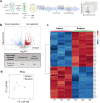
Spatial cell organization and biofabrication of microcapillary networks in vitro has a great potential in tissue engineering and regenerative medicine. This study explores the impact of local cell density enhancement achieved through an innovative sound-based patterning on microcapillary networks formation and their proteomic profile. Human umbilical vein endothelial cells (HUVEC) and human pericytes from placenta (hPC-PL) were mixed in a fibrin suspension. The mild effect of sound-induced hydrodynamic forces condensed cells into architected geometries showing good fidelity to the numerical simulation of the physical process. Local cell density increased significantly within the patterned areas and the capillary-like structures formed following the cell density gradient. Over five days, these patterns were well-maintained, resulting in concentric circles and honeycomb-like structures. Proteomic analysis of the pre-condensed cells cultured for 5 days, revealed over 900 differentially expressed proteins when cells were preassembled through mild-hydrodynamic forces. Gene ontology (GO) enrichment analysis identified cellular components, molecular functions, and biological processes that were up- and down-regulated, providing insights regarding molecular processes influenced by the local density enhancement. Furthermore, we employed Ingenuity Pathway Analysis (IPA) to identify altered pathways and predict upstream regulators. Notably, VEGF-A emerged as one of the most prominent upstream regulators. Accordingly, this study initiates the unraveling of the changes in microcapillary networks at both molecular and proteins level induced by cell condensation obtained through sound patterning. The findings provide valuable insights for further investigation into sound patterning as a biofabrication technique for creating more complex microcapillary networks and advancing in vitro models.
Keywords: Bio-assembly; Cell density enhancement; Microcapillary networks; Proteomic analysis; Sound patterning.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Enderle J., Bronzino J. Academic Press; 2012. Introduction to biomedical engineering.
-
- Miller K.L., et al. Rapid 3D BioPrinting of a human iPSC-derived cardiac micro-tissue for high-throughput drug testing. Organs-on-a-Chip. 2021;3
LinkOut - more resources
Full Text Sources
