Evaluation of cyanotoxin L-BMAA effect on α-synuclein and TDP43 proteinopathy
- PMID: 38596666
- PMCID: PMC11002123
- DOI: 10.3389/fimmu.2024.1360068
Evaluation of cyanotoxin L-BMAA effect on α-synuclein and TDP43 proteinopathy
Abstract
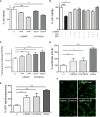
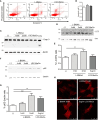
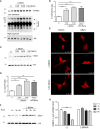
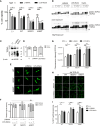
The complex interplay between genetic and environmental factors is considered the cause of neurodegenerative diseases including Parkinson's disease (PD) and Amyotrophic Lateral Sclerosis (ALS). Among the environmental factors, toxins produced by cyanobacteria have received much attention due to the significant increase in cyanobacteria growth worldwide. In particular, L-BMAA toxin, produced by diverse taxa of cyanobacteria, dinoflagellates and diatoms, has been extensively correlated to neurodegeneration. The molecular mechanism of L-BMAA neurotoxicity is still cryptic and far from being understood. In this research article, we have investigated the molecular pathways altered by L-BMAA exposure in cell systems, highlighting a significant increase in specific stress pathways and an impairment in autophagic processes. Interestingly, these changes lead to the accumulation of both α-synuclein and TDP43, which are correlated with PD and ALS proteinopathy, respectively. Finally, we were able to demonstrate specific alterations of TDP43 WT or pathological mutants with respect to protein accumulation, aggregation and cytoplasmic translocation, some of the typical features of both sporadic and familial ALS.
Keywords: ALS; L-BMAA; PD; TDP43; cyanotoxins; α-synuclein.
Copyright © 2024 Sini, Galleri, Ciampelli, Galioto, Padedda, Lugliè, Iaccarino and Crosio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

