Non-autonomous regulation of neurogenesis by extrinsic cues: a Drosophila perspective
- PMID: 38596708
- PMCID: PMC10913833
- DOI: 10.1093/oons/kvac004
Non-autonomous regulation of neurogenesis by extrinsic cues: a Drosophila perspective
Abstract
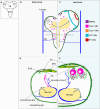
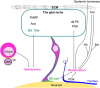
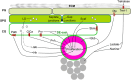
The formation of a functional circuitry in the central nervous system (CNS) requires the correct number and subtypes of neural cells. In the developing brain, neural stem cells (NSCs) self-renew while giving rise to progenitors that in turn generate differentiated progeny. As such, the size and the diversity of cells that make up the functional CNS depend on the proliferative properties of NSCs. In the fruit fly Drosophila, where the process of neurogenesis has been extensively investigated, extrinsic factors such as the microenvironment of NSCs, nutrients, oxygen levels and systemic signals have been identified as regulators of NSC proliferation. Here, we review decades of work that explores how extrinsic signals non-autonomously regulate key NSC characteristics such as quiescence, proliferation and termination in the fly.
Keywords: Drosophila; neuroblast; niche.
© The Author(s) 2022. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures


Similar articles
-
The Drivers of Diversity: Integrated genetic and hormonal cues regulate neural diversity.Semin Cell Dev Biol. 2023 Jun;142:23-35. doi: 10.1016/j.semcdb.2022.07.007. Epub 2022 Jul 29. Semin Cell Dev Biol. 2023. PMID: 35915026 Review.
-
Systemic and local cues drive neural stem cell niche remodelling during neurogenesis in Drosophila.Elife. 2018 Jan 4;7:e30413. doi: 10.7554/eLife.30413. Elife. 2018. PMID: 29299997 Free PMC article.
-
Protein S Regulates Neural Stem Cell Quiescence and Neurogenesis.Stem Cells. 2017 Mar;35(3):679-693. doi: 10.1002/stem.2522. Epub 2016 Nov 8. Stem Cells. 2017. PMID: 27753164
-
The vasculature as a neural stem cell niche.Neurobiol Dis. 2017 Nov;107:4-14. doi: 10.1016/j.nbd.2017.01.010. Epub 2017 Jan 26. Neurobiol Dis. 2017. PMID: 28132930 Review.
-
Regulation of neurogenesis in the adult and aging brain.Curr Opin Neurobiol. 2018 Dec;53:131-138. doi: 10.1016/j.conb.2018.07.006. Epub 2018 Aug 2. Curr Opin Neurobiol. 2018. PMID: 30077888 Review.
References
-
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
