A Clinical Trial of High-Dose Growth Hormone in a Patient With a Dominant-Negative Growth Hormone Receptor Mutation
- PMID: 38597155
- PMCID: PMC11479683
- DOI: 10.1210/clinem/dgae244
A Clinical Trial of High-Dose Growth Hormone in a Patient With a Dominant-Negative Growth Hormone Receptor Mutation
Abstract
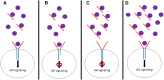
Context: Rare patients with short stature and growth hormone (GH) resistance have dominant-negative variants in the GH receptor. We describe a patient with GH resistance due to elevated levels of GH binding protein and demonstrate the potential for a precision medicine intervention.
Objective: To determine whether high-dose GH can overcome GH resistance in this specific patient resulting in normal insulin-like growth factor (IGF)-1 levels and improved growth rates.
Methods: Single patient trial of ascending doses of GH followed by a dose stable phase: total 12 months of treatment. The patient has a heterozygous variant in the GH receptor resulting in elevated levels of GH binding protein manifesting as GH resistance and severe short stature. Daily subcutaneous GH was administered, starting at 50 µg/kg/day and escalating to 250 µg/kg/day until goal IGF-1 achieved. The subject continued on 250 µg/kg/day for a total treatment duration of 12 months. The primary outcome measure was the dose of GH required to achieve an IGF-1 level above the midpoint of the normal range. Secondary endpoints included height velocity and the change in height SDS during the first year of treatment.
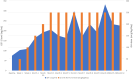
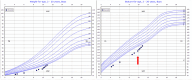
Results: A dose of GH of 250 µg/kg/day achieved the target IGF-1 level. The patient's annualized height velocity was 8.7 cm/year, an increase of 3.4 cm/year from baseline, resulting in a 0.81 SD gain in height.
Conclusion: A precision medicine approach of extremely high dose GH was able to overcome GH resistance in a patient with a dominant-negative variant in the GH receptor resulting in elevated GH binding protein levels.
Keywords: growth hormone; growth hormone receptor; growth hormone resistance; precision medicine.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures
References
-
- Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008;93(2):352‐357. - PubMed
-
- Bang P, Polak M, Woelfle J, Houchard A. Effectiveness and safety of rhIGF-1 therapy in children: the European increlex® growth forum database experience. Horm Res Paediatr. 2015;83(5):345‐357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

