Pharmacokinetics and Pharmacodynamics of Inhaled Nicotine Salt and Free-Base Using an E-cigarette: A Randomized Crossover Study
- PMID: 38597729
- PMCID: PMC11417154
- DOI: 10.1093/ntr/ntae074
Pharmacokinetics and Pharmacodynamics of Inhaled Nicotine Salt and Free-Base Using an E-cigarette: A Randomized Crossover Study
Abstract
Introduction: Popular "pod-style" e-cigarettes commonly use nicotine salt-based e-liquids that cause less irritation when inhaled and can deliver higher nicotine concentrations than free-base nicotine. This study investigated the pharmacokinetic and pharmacodynamic effects of different nicotine formulations (salt vs. free-base) and concentrations that might influence systemic nicotine absorption and appeal of e-cigarettes.
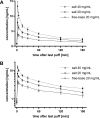
Aims and methods: In this randomized, double-blind, within-subject crossover study, 20 non-nicotine-naïve participants were switched among three e-liquids (free-base nicotine 20 mg/mL, nicotine salt 20 mg/mL, nicotine salt 40 mg/mL) using a refillable pod system and a standardized vaping protocol (one puff every 30 seconds, 10 puffs total). Serum nicotine concentrations and vital signs were assessed over 180 minutes; direct effects, craving, satisfaction, withdrawal, and respiratory symptoms were measured using questionnaires. CYP2A6 genotypes and the nicotine metabolite ratio were also assessed.
Results: Eleven (55%) participants were male and the median age was 23.5 years (range 18-67). All three formulations differed significantly in peak serum nicotine concentration (baseline adjusted Cmax, median (range): 12.0 ng/mL (1.6-27.3), 5.4 ng/mL (1.9-18.7), and 3.0 ng/mL (1.3-8.8) for nicotine salt 40 mg/mL, nicotine salt 20 mg/mL and free-base 20 mg/mL, respectively). All groups reached Cmax 2.0-2.5 minutes (median) after their last puff. Differences in subjective effects were not statistically significant. No serious adverse events were observed.
Conclusions: Free-base 20 mg/mL formulations achieved lower blood nicotine concentrations than nicotine salt 20 mg/mL, while 40 mg/mL nicotine salt yielded concentrations similar to cigarette smoking. The findings can inform regulatory policy regarding e-liquids and their potential use in smoking cessation.
Implications: Nicotine salt formulations inhaled by an e-cigarette led to higher nicotine delivery compared to nicotine-free-base formulations with the same nicotine concentration. These findings should be considered in future regulatory discussions. The 40 mg/mL nicotine salt formulation showed similar nicotine delivery as combustible cigarettes, albeit at concentrations over the maximum limit for e-liquids allowed in the European Union. Nicotine delivery resembling combustible cigarettes might be beneficial for smokers willing to quit to adequately alleviate withdrawal symptoms. However, increased nicotine delivery can also pose a public health risk, raising concerns about abuse liability, especially among youth and nonsmokers.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco.
Conflict of interest statement
CBE received honoraria for conferences from Forum pour la formation médicale, Janssen-Cilag, Lundbeck, Otsuka, Sandoz, Servier, Sunovion, Sysmex Suisse AG, Takeda, Vifor-Pharma, and Zeller in the past 3 years. EL reports academic institution research support for investigation of the pharmacology and toxicology of e-cigarettes. NLB serves as a consultant to Achieve Life Sciences, which is developing new smoking cessation medications, and has been an expert witness in litigation against tobacco companies. VvdV declares that her spouse is employed by Philip Morris International. All authors declare that they have no conflict of interest in relation to the content of this work.
Figures

References
-
- Dempsey D, Tutka P, JacobP, 3rd, et al. . Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. - PubMed

